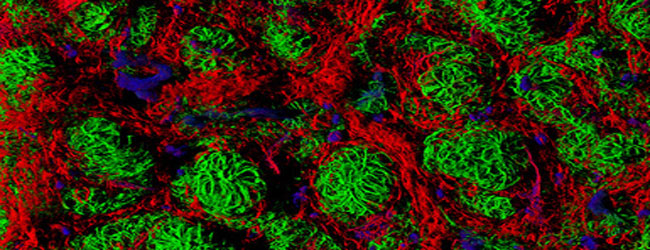
Spectral Unmixing in Fluorescence Microscopy
When we are doing a double labelling, it makes good sense to choose two fluorophores with spectra widely spaced apart to avoid mixing. Why combine GFP with Rhodamine, when you can combine it with Texas Red?