The electron microscope (EM) – where electrons, rather than photons, make the image – fell out of fashion for a while, but it has come back refreshed. Modern electron microscopes cost less, use less electricity, and are generally easier to maintain than the older models, so it is likely that you can get your hands on one. Read on to learn more about this technique, and how to implement it in your research.
Electron Microscopy: What Is It Good For?
There are two main types of EM – transmission EM (TEM) and scanning EM (SEM). If you need to get an idea of the surface or composition of your sample, you would choose SEM. However, if you are more interested in the inner structure of the sample (think: cross-section), then you would choose TEM.
TEM has a better than 50 pm resolution and a magnification level of up to about 50,000,000. [1] You should be impressed: most light microscopes are limited to about 200 nm resolution and useful magnifications below 2000× because of diffraction. SEM is also capable of a near-atomic resolution (approximately 3800 – to 4500 pm), which is enough to distinguish between alpha-helix and beta-sheet in a viral protein. [2] However, the maximum magnification level of SEM only reaches 2,000,000.
While some biologists may only see EM as being useful in the imaging of microstructures such as bacterial flagella, I am here to let you know that it can do so much more. For instance, for all you budding zoologists, EM can be successfully used to image microstructures such as the finest details of carapaces of insects, feathers, and insect eyes.
EM Modifications
On the other side of the scale, a modification of EM called cryo-EM is used to resolve macromolecular complexes’ structures with a resolution close to X-ray crystallography. This is important because it saves time on optimizing conditions for crystal growth. And you know that some crystals stubbornly refuse to grow, and some refuse to diffract.
Because of the superb resolution, EM is indispensable if you work with viruses. Remember the pictures of coronavirus budding from the human cells? The same principle applies to bacteria that are close to the resolution limit of the light microscope.

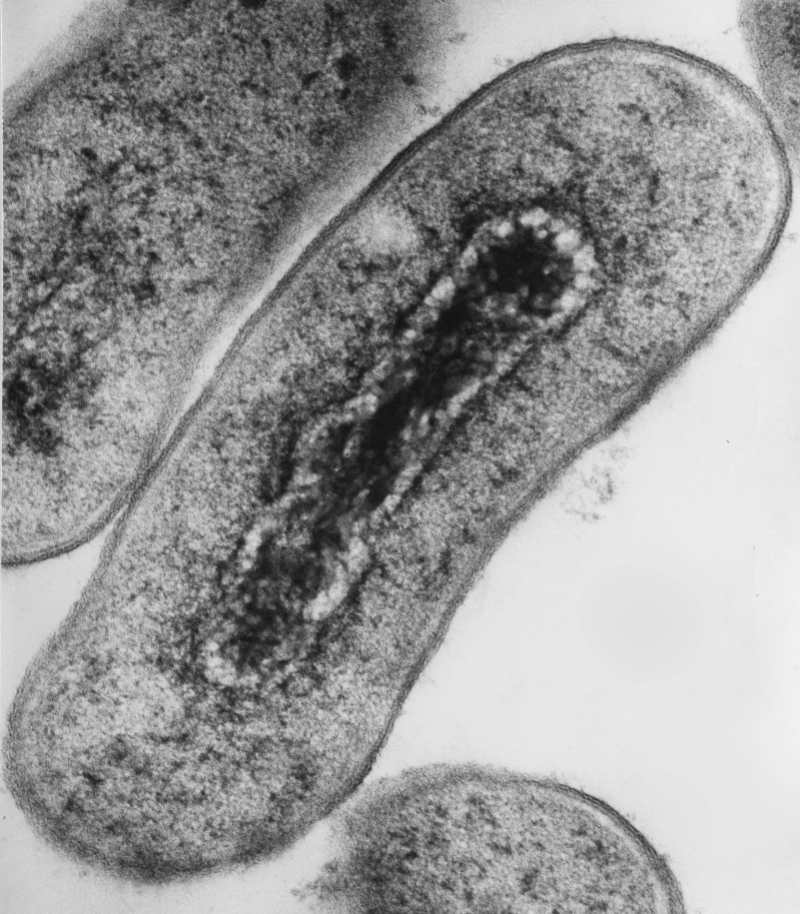
Smaller eukaryotic cells such as yeast also benefit from EM use, and for large mammalian cells, you can concentrate on the subcellular structures. Furthermore, you can pinpoint the localization of your protein of interest using antibodies conjugated with electron-dense material. How cool would it be to decorate your protein with gold nanoparticles?
How to Treat Your Sample
The electron beam of EM works in a vacuum, which means that you won’t be able to work with a “live” or even “wet” specimen. Your specimen will need fixation, usually done by a trained technical specialist who will also help with EM imaging.
If you think about it, EM pictures are always in black and white. Why? Unlike light waves, electrons don’t have a wavelength and, therefore, are absent of color. Color electron photographs that you see sometimes are false colors attributed by software to different shading.
There are several methods of sample preparation for EM. The method of choice will depend on your sample. But consider the following possibilities:
- Classic “metal shadowing” – heavy metal such as platinum evaporates from an overhead electrode metal onto the surface of a biological sample. The variations in the thickness of the metal are seen as variations in brightness and contrast in the electron microscope image.
- “Staining” with metals – using lead or tungsten to scatter imaging electrons, providing contrast between different structures.
- “Chemical fixation” – to stabilize the specimen’s macromolecular structures by chemical crosslinking of proteins with aldehydes such as formaldehyde, and lipids with osmium tetroxide.
- “Dehydration and embedding” – the sample is dehydrated and embedded in a mixture of a solvent such as acetone and epoxy resin. The embedded sample can be sectioned, allowing for EM-imaging of each section and reconstitution of the 3D sample structure.
- ““Negative stain” – suspension of small biological objects such as viruses and bacteria is mixed with an electron-opaque solution such as ammonium molybdate, uranyl acetate. The mixture is applied to a suitably coated EM grid, blotted, allowed to dry, and used immediately. This is a relatively rapid method.
- “Cryopreservation” – sample is rapidly frozen, which allows it to retain macromolecular complexes in their native configurations. EM on frozen samples (cryoelectron microscopy) branched out of the “classical EM” that mostly uses metal staining and shadowing and became a discipline in its own right. [3] Find our article about cryoelectron microscopy for more details and discover 5 crucial considerations for cryo-EM sample prep.
If you can locate an electron microscope, I recommend arranging a meeting with the person responsible for it. They are usually very helpful and eager to collaborate. Just describe your object of study and ask what electron microscopy can do for you. I bet that whatever you study, you can get new exciting results from this classical method.
Sources:
- Erni, R, et al. (2009). Atomic-Resolution Imaging with a Sub-50-pm Electron Probe. Physical Review Letters. 102 (9): 096101.doi:10.1103/PhysRevLett.102.096101.
- Zhou H. Z. (2008) Towards Atomic Resolution Structural Determination by Single-Particle Cryo-Electron Microscopy. Review Curr Opin Struct Biol. 18(2):218-28. doi: 10.1016/j.sbi.2008.03.004.
- Dillard, RD, et al. (2018) Biological Applications at the Cutting Edge of Cryo-Electron Microscopy. Microsc Microanal. 24(4):406-419. doi: 10.1017/S1431927618012382.





