This article is for anyone who has ever wondered, “How do I know when my bacterial culture is ‘done’ growing? When should I harvest my cells?”. We’ll talk you through the bacterial growth curve and show you how to check what stage your culture is in using OD600 measurements.
Bacterial Growth Curves: A Review
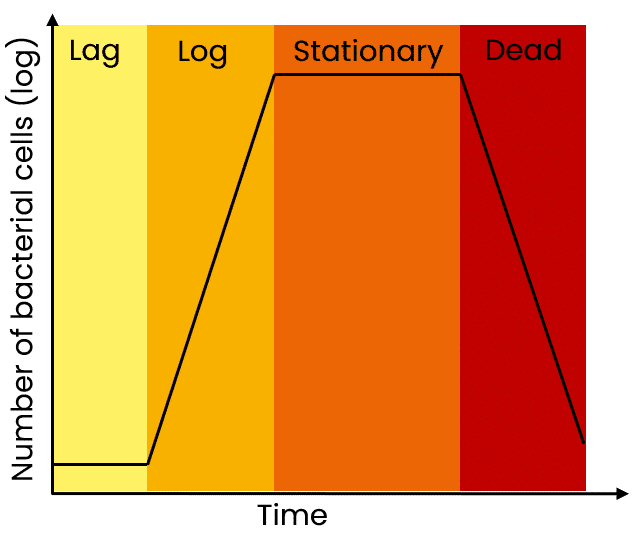
Let’s start by reviewing the four basic phases of a bacterial growth curve (Figure 1):
- lag phase
- log (exponential) phase
- stationary phase
- death phase.
A fresh inoculation of bacteria into culture medium starts out in the lag phase, where cells are metabolically active but not dividing. During this time, cells are adapting and adjusting to their new environment.
The bacterial culture then enters the log phase, where cells rapidly divide (normally by binary fission) and double in number over a given period of time (e.g. E. coli can double every 20 minutes in log phase).
Growth during this phase is exponential and metabolic activity is uniform across cells; bacteria in this phase are also most susceptible to antibiotics, for example, making it the ideal phase for research work.
When a maximum cell density in a liquid culture is reached, cells enter the stationary phase.
During this phase, there is no overall increase in the cell population—cells that die are replaced by newly dividing ones, but the overall number of live cells stays the same.
If you’re working with spore-forming bacteria, this may be the stage where vegetative cells sporulate or form new spores.
And finally, the death (or decline) phase occurs due to a variety of factors, including a lack of nutrition or oxygen, a change in media pH as a result of cell metabolism, or an accumulation of toxic cellular waste.
Using OD600 Measurements to Determine Your Bacterial Culture Growth Stage
Measuring the OD600, or the optical density (OD) at 600 nm, is the easiest way to gauge the bacterial culture growth stage. Optical density measures the degree of light scattering caused by the bacteria within a culture; the more bacteria there are, the more the light is scattered.
The 600-nm wavelength is specifically chosen for bacterial OD measurements because unlike UV wavelengths, 600 nm is not harmful to the culture. This wavelength is also not usually absorbed by yellow-ish media like TSB (tryptic soy broth) and LB (lysogeny broth).
By monitoring the rate of increase in OD600, you can identify the lag, log, and stationary phases of a bacterial culture.
So how do you take this measurement? For a simple suspension of cells in liquid, you first need to zero or “blank” the spectrophotometer with fresh, uninoculated medium to subtract any absorption contribution the medium has on the measurement.
Then, take a sample of your culture and measure the OD600.
When measuring the OD600, there are a few things to watch out for:
- When you pull a culture sample, ensure that you’ve mixed it well and take the measurement right away, since cells can begin to settle within a minute or so, which leads to inaccurate results.
- If your bacteria form biofilms or aggregates in solution, this will severely affect the accuracy and precision of your measurement. In this situation, you might need to sonicate or further process the culture to break apart these ‘clumps’. Check the literature regarding the bacterial strain you are working with to avoid or negate this problem.
- Most importantly… an OD reading of > 1 is not accurate! OD readings this high are beyond the dynamic range of most spectrophotometers, meaning that the readings do not increase linearly as cell concentration increases. If you do obtain an OD reading higher than 1, dilute your sample 2-fold or more until it reaches an OD600 of < 1.
OD600 is Not an Absolute Value
The OD value represents the amount of light that is scattered by your sample. But that value is affected by the intensity of the light beam in the spec, and the spec design. This means that similar samples will give completely different OD values in different specs (owing to the specs having different bulbs), or even in the same spec over time, as the beam intensity reduces with the age of the bulb.
So, recording an OD value in your lab book doesn’t really mean anything as this number is as much dependent on your spec as on the density of your culture.
Calculating the Cell Density from OD600
What you really want to know from an OD600 reading is the density of the cells, e.g. in cells/mL. And to get this you need a standard curve. In other words, like any other spec-based experiment you will ever perform, you need to calibrate the absorbance value against the number you actually want to know.
For some reason, people don’t seem to remember this for OD600. I can’t think of any other experiment where people record the absorbance value as an absolute number as they do for OD600, but maybe you can think of some—tell me in the comments :).
Constructing a standard curve for OD600 is a bit tedious, but it is a good exercise to go through every 6 months or so (and for each spec you use).
Calibrating Your OD600 Measurements
Start by making a suspension culture of the cells you are interested in, then diluting it to obtain a series of samples with ODs of =2, 1, 0.8, 0.6, 0.4, 0.2, and 0.1 on your spec. (NB: don’t make serial dilutions as these are very inaccurate.)
For each OD you then want to know the cell density in cells/mL. So for each OD, make dilutions of 1 in 1×10^7, 1×10^6, and 1×10^5, then plate 1 mL of each onto suitable plates and grow them up.
Then count the number of colonies formed on the dilution that gives the most appropriate number and multiply up by the dilution factor to obtain the number of cells/mL in the original sample. These values can then be used to construct a calibration curve of OD vs. cells/mL.
The conversion factor for your spec (the number of cells/mL represented by 1 OD unit) will be equal to the gradient of the linear portion of the curve (normally up to about OD=1). You can now use this factor to convert your OD reading to cells/mL and as long as you calibrate whatever spec you are using in the future, this number will be absolute and comparable between experiments, specs, and years.
Just When You Thought It All Made Sense…
A word of warning though, since the OD of a sample is dependent on the size and shape of the particles in it, different cell lines can have completely different relationships between OD and cells/mL.
This means that a separate calibration will be needed for each cell type you use, which is tedious, but better than recording meaningless and arbitrary numbers in your lab book.
Estimating OD600 by Eye
If you work with flasks to grow bacteria on a regular basis, it’s helpful to train yourself to estimate OD by eye. This way, you can save valuable time by skipping OD600 checks when flasks are clearly not within the OD range you are looking for. It doesn’t replace OD600 entirely but can help you cut down on the number of times you need to measure the OD600.
You might also want to consider using a microplate reader to increase the throughput of your OD measurements.
Other Methods of Monitoring Bacterial Culture Growth and Metabolism
Though monitoring growth using a bacterial culture’s OD600 is a tried and true method, there are several other ways to assess the growth and metabolism of bacterial cultures. You may find that some of these factors are more relevant or useful for your specific application, too!
Imaging
By taking samples of your culture and checking them out under a microscope, you can begin to get a sense of the approximate concentration of live cells in your culture.
Bonus: you can compare images of cells to see if there are different morphologies from batch to batch, or how many vegetative cells are in a spore-forming culture.
Dissolved Oxygen and Carbon Dioxide
Consider beer (made from yeast), kombucha (made from yeast and various bacteria), and other enjoyable beverages fermented by microbes. As the fermentation progresses in these examples, oxygen (O2) is consumed and carbon dioxide (CO2) is formed.
The same process happens in an aerobically growing bacterial culture! Dissolved O2 and CO2 probes are commonly used in bioreactors. If you want a less invasive option, you can get optical sensors (and patches) that use LED fluorescence to monitor dissolved O2 from outside a flask!
pH
Most bacterial species lower the pH of their media over time, including E. coli. For some species, acid production can actually limit a culture’s maximum growth and viability. Like oxygen sensors, methods exist to continuously monitor pH non-invasively in flasks.
Sugar Analysis
With certain instruments, getting a fast and reliable measurement of residual sugars from your growth medium is possible.
For instance, if the primary carbon source in your medium is glucose, obtaining a glucose measurement midway through bacterial growth can provide a sense of where the culture is in the growth curve.
So When SHOULD You Harvest Your Culture?
In short, it depends entirely on what the end goal is for the culture.
For instance, I used to work extensively growing and inducing E. coli to overexpress non-native proteins, which would then be purified from culture.
I often found that the quality and quantity of protein I obtained from the culture depended on the phase cells were in when I harvested them.
In other cases, however, the goal is to simply harvest as many cells as possible.
Once you know the specific growth phase at which you need to harvest cells, be sure to perform a couple of test runs (including measurements) to make sure you are correct in your assessment of the bacterial growth timing.
This will give you a strong sense of how long it takes for your culture to be ready to harvest and provides historical data for comparing bacterial culture growth curves.
Always start from a smaller “seed” culture to get reliable and consistent growth in flasks. Finally, test your cell culturing techniques a few times until you’re comfortable to reduce the risk of contamination. One method to save time and get reproducibility when growing the same bacterial cultures routinely is to create a bacterial cell culture bank.
From the relatively simple OD600 measurement to metabolite analysis, there are a plethora of ways to keep tabs on your growing bugs in the lab. Because the growth stage affects much within a culture, knowing how to accurately measure this parameter is a key technique in any bacterial lab!
Do you have any tips for OD600 measurements or bacterial culture growth monitoring? Tell us in the comments below!
Originally published May 29, 2018. Reviewed and updated July 2021.
During the review process, this article was merged with another article from Nick Oswald.