A Beginner’s Guide to Culturing Mouse Embryonic Stem Cells

There is something undeniably special about embryonic stem cells (ESCs) and not just because they can produce every cell type in the adult body. In vivo, ESCs are a transitory state of early development, which has been captured indefinitely in vitro. Whether you are a hardened cell culture enthusiast or have just graduated from the school of 3T3 fibroblasts, there are a few important points to remember when culturing ESCs. Here, I cover specifically mouse ESCs because human ESCs behave differently and I only address aspects of mESC culture and not methods for deriving them from early embryos.
What Conditions Should I Use?
Mouse ESCs were first derived in 1981. Since then, they have been largely cultured with fetal calf serum and leukemia inhibitory factor (LIF), which is a cytokine that maintains pluripotency by activating the transcription factor STAT3. Then, in 2008, Austin Smith’s group showed that pluripotency can be maintained in the absence of serum and growth factors by two small chemical inhibitors, PD0325901, which inhibits mitogen-activated protein kinase, and CHIR99021 which inhibits glycogen synthase kinase-3. Although not strictly necessary, LIF is also used for maximal pluripotency.
Ok, so which system is best for me? Debate is ongoing between the two parties, although pretty compelling evidence shows that ‘2i+LIF’ is best to maintain pluripotency. First, these conditions enable mESCs to be derived from many mouse strains and rat embryos that do not yield ESCs under conditions of serum. Furthermore, cells cultured in ‘2i+LIF’ have lower levels of DNA methylation than those cultured in ‘serum+LIF’ and they express important pluripotency factors like Nanog homogenously (i.e. in every cell), which isn’t the case for mESCs grown in serum. But here comes the catch. These inhibitors are expensive, so ‘2i+LIF’ conditions aren’t suitable for experiments requiring large scale production, unless your lab did exceptionally well with grant applications last year.
It may be useful to use both conditions: you could perform most experiments in ‘serum+LIF’, but use a small amount of ‘2i+LIF’ to check that your cells behave the same way under these conditions. If you are using serum then don’t just take the first bottle you find in the freezer. Every serum is different and mESCs may start to differentiate at a high rate in culture, even in the presence of LIF, if you don’t choose the right one. If your lab does not routinely work with ESCs, then it is wise to purchase serum that has been specifically tested for ESC culture. When culturing ESCs in serum use DMEM and 15% FBS, whereas in 2i, use N2B27 medium, 3 ?M CHIR99021 and 1 ?M PD0325901 (103 units/ml of LIF should be added to both).
Do I Need Antibiotics?
The short answer is no. Unless you are deriving mESCs from embryos, there is no need for antibiotics, which should never be used as a substitute for good aseptic technique.
Do I Need Feeders?
Feeders are mitotically inactivated mouse embryonic fibroblasts (MEF) that support the growth of mESCs. Some mESCs lines grow quite well without feeders whereas others proliferate poorly without them. Check the specifications for your cell line. Feeders are not required in conditions of 2i+LIF. Feeders are typically prepared by expanding primary MEF and inactivating them with mitomycin C, which creates crosslinks in DNA. It is wise to make a large stock of feeders in one go, but this involves a lot of cell culture, so get someone to help you. Try to aim for 3×106 cells per vial when freezing, because this is sufficient to cover a T75 flask. Feeders can be thawed in advance or with mESCs, because they adhere more quickly to the plate. If you are growing cells without feeders then you should coat your plates with 0.1% gelatin to help mESCs to adhere.
Passaging mESCs
ESCs are really cool. You can turn them into beating embryoid bodies or neuronal precursors with sprouting axons. But they need your love and attention. Their cell cycle checkpoints differ from those of differentiated cells and mESCs transit through G1 phase in only 1-2 hours. Thus, mESCs can complete a full cell cycle in 8–12 hours, which is a lot quicker than non-transformed, early passage murine cells (24–36 hours). Unfortunately, this means that mESCs need to be passaged every other day, regardless of meetings, conferences and weekends. Many mESCs need their media changing every day (you may see it turn from red to orange/yellow in only 24 hours). Share the burden with your colleagues. Everyone is owed at least one favor.
Mouse ESCs are passaged much like other cell lines. Some protocols recommend using less harsh proteolytic solutions like accutase, but low volumes of trypsin are generally ok for splitting mESCs. There is no need to separate mESCs from feeders when passaging, but you do need to remember to thaw enough feeders for the new flasks. A 1:4 to 1:8 split works well for most cell lines.
Separating mESCs from Feeders
So you are finally ready to do some cool experiments with your mESCs but they may be stuck on a layer of feeder cells. Gentle proteolytic agents like accutase or collagenase (type IV) can detach mESCs colonies without disturbing feeder cells, but if you prefer a cheaper method, then mESCs can be separated from feeders by taking advantage of differences in cell adhesion.
For each flask you have, prepare two plates coated with 0.1% gelatin (make sure you use plates and not flasks because they are easier to manipulate). Dissociate cells with trypsin. Then, distribute the cell suspension onto one plate with enough media to cover the bottom. Put the plate back in the incubator for 15 minutes. Tap the plate gently to dislodge the cells that have not adhered to the bottom, and gently pipette the media containing suspended cells. Repeat the process. You should now be left with media containing a highly pure population of mESCs, because feeder cells adhere quickly to culture dishes and are removed during the plating process. Don’t worry about any leftover feeder cells. At confluence, mESCs can outnumber feeder cells by more than 20:1, so a few contaminating feeder cells after the depletion process won’t affect your experiments.
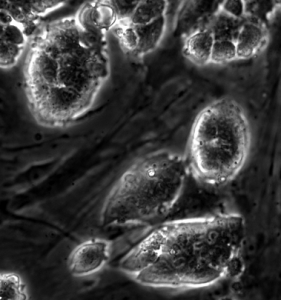
Figure 1. Image of mESCs cultured with feeder cells. Image courtesy of Antonio Romito.
Always remember, a happy mESC is a pluripotent mESC. Your mESCs should form compact colonies on feeder cells (Figure 1.). If they are flat at the edges and you can make out individual cells then they are probably differentiating. Treat them well, and you can make just about any cell type you want with them.
Do you have top tips for culturing mESCs? Leave them in the comments below.
5 Comments
Leave a Comment
You must be logged in to post a comment.
Very good description. One minor thing, when separating ES cells from feeders, use a non-gelatin coated dish as this makes it more difficult for ES cells to settle. Plate then ES cells that are still in suspension after 15 – 20 min on a gelatinised dish. This will result in a better separation of the ES cells from feeders.
Thanks for this valuable knowledge! Are there any papers you can refer to for the “Separating mESCs from Feeders” part?
exactly the information I was looking for all in one easy read. Thank you!
Very helpful article.
[…] The sky’s the limit: This form of culturing enhances other techniques such as 3D cell culturing using scaffolds and magnetic levitation. The title of this point is so apt because you could theoretically grow anything from spinal tissue to organs! As you can imagine co-culturing is a hot topic in murine and human stem cell research. For more info on co-culutirng murine stem cells, see our BitesizeBio article! […]