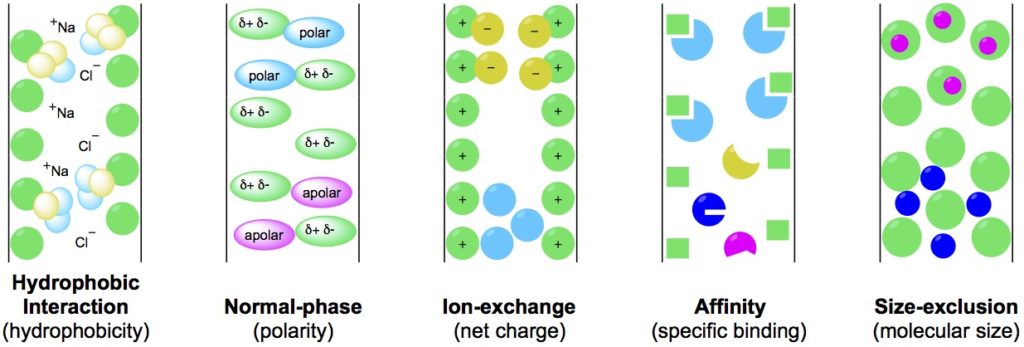
Sample purity. We all need it. We all seek it.
In biosciences, a vast amount of time is invested in purifying samples. Pure DNA for transformation and transfection. Pure RNA for sequencing. Pure proteins for assays and structural studies.
Sample purity is achieved using chromatography—the process of separating components of a mixture.
So, how long do you spend performing chromatography? What methods do you use? And how often does it fail and need optimizing?
The chances are the answer to all those questions is “a lot.”
Let’s have a quick rundown of how chromatography works. You can then apply your developed understanding of it to save time, improve your sample purity, and achieve more successful experiments.
Basic Chromatography Terms Defined
As dry as a ship’s biscuit, but to understand how chromatography works, we need to define some key terms. These are common to all chromatography methods and will help you communicate your experiments and aims.
Stationary Phase
The stationary phase is an immobilized species that interacts chemically or physically with the molecules you wish to separate. Usually, the stationary phase consists of chemical functional groups covalently linked to a polymer. This polymer is in turn mechanically linked to the chromatography column.
Mobile Phase
The mobile phase is a solvent or buffer system that carries the molecules you wish to separate through the column in a manner that allows the molecules to encounter the stationary phase.
In a successful chromatography experiment, the target molecules and impurities have different affinities for the stationary phase. Thus, one spends a longer duration on the column than the other, allowing separation to occur.
Eluent
The eluent is a specific chemical component of the mobile phase that causes the target molecules to elute from the column. Please don’t confuse it with the mobile phase itself.
For example, in nickel-affinity chromatography, imidazole is the eluent. The buffer system in which the imidazole is dissolved, is the mobile phase.
Analyte
The analyte is the target molecule that you wish to separate from impurities.
Matrix
The matrix, which is also called the sample matrix, is the combined mixture of analytes and impurities that you pass over the chromatography column.
Retention Time and Volume
Retention time and volume refer to the duration that an analyte spends on the chromatography column.
Retention time is usually measured relative to the time of analyte injection onto the column.
Retention volume is usually measured relative to the amount of mobile phase pumped onto the column between analyte injection and analyte detection.
How Chromatography Works: The Fundamental Principle
Separation in column chromatography relies on differences.
Specifically, differences in the chemical and physical properties of molecules.
Molecules vary in size, charge, polarity, and solubility. In chromatography, these differences are exploited to distribute molecules between a stationary phase and a mobile phase.
However, some molecules only differ slightly from one another. Also, the matrix from which you wish to separate your target molecule might contain thousands of different chemicals.
Therefore, it’s not possible to have a single separation method that works for every target molecule.
What that means is there are many types of chromatography. The five that we will discuss in this article are:
- Hydrophobic interaction chromatography;
- Normal/reverse-phase chromatography;
- Ion-exchange chromatography;
- Affinity chromatography;
- Size-exclusion chromatography.
These methods each harness different molecular properties to separate analytes from crude sample matrixes. Return to the graphical representation of these methods (Figure 1) for reference as we move through this article.
1. How Hydrophobic Interaction Chromatography Works
Hydrophobic interaction chromatography separates molecules based on their hydrophobicity.
The stationary phase possesses hydrophobic groups that interact with the hydrophobic regions of analyte molecules. But they have to encounter each other first.
Remember that oil and water don’t mix? That’s because oil is hydrophobic—it’s not attracted to water because there are no polar regions within the oil to bind to the polar water molecules.
The same principle applies to a hydrophobic stationary phase and water. There are no interactions between them.
Instead, a network of polar hydrogen bonds forms in the mobile phase. This network surrounds and shields the hydrophobic groups on the column stationary phase from the analytes passing through it.
This prevents them from binding to the stationary phase.
To enable binding between the hydrophobic stationary phase and the analytes, the shield of polar hydrogen bonds must be broken by introducing a chaotropic salt.
The ions introduced to the mobile phase interact with water, break the polar hydrogen bond network, and exposes the hydrophobic groups to the stationary phase.
This allows binding to occur between the stationary phase and the analytes. And the exact composition of the mobile phase and choice of stationary phase can be tailored to achieve separate target analytes.
In summary:
- Analytes bind to the column in a high salt concentration mobile phase.
- Analytes are eluted using a low salt concentration/chaotropic mobile phase.
2. How Normal/Reversed-Phase Chromatography Works
Remember that like attracts like?
Normal/reversed-phase chromatography separates molecules by their polarity.
The stationary phase contains either highly polar (normal-phase) or highly non-polar (reverse-phase) functional groups that interact with analytes proportionate to their polarity.
If we go back to the concept of oil and water not mixing, we could also say that because water is highly polar and oil is highly non-polar, they don’t attract each other. Instead, oil molecules are attracted to other oil molecules, and water molecules are attracted to other water molecules.
In other words, polar attracts polar, and non-polar attracts non-polar.
In normal-phase chromatography, the stationary phase is more polar than the mobile phase. Since polar molecules are retained in the column, non-polar molecules elute before polar ones.
For reversed-phase chromatography, things are, well, the reverse. You use a non-polar stationary phase that retains non-polar compounds leaving the polar molecules to elute first.
3. How Ion-Exchange Chromatography Works
Opposites can attract too.
Ion-exchange chromatography separates molecules based on their ionic interactions with one another. The stationary phase is a resin that contains ionic functional groups that bind to analytes of the opposite charge.
So, if you wish to purify your negatively charged analyte, you pass it through a positively charged resin. The molecules that don’t bind (positively charged), or bind weakly (slightly negatively charged), elute first while the analyte remains bound to the column.
Since the analyte is negatively charged, this process is called anion-exchange chromatography.
If you wish to purify a positively charged analyte, you pass it through a negatively charged resin. The molecules that don’t bind (negatively charged), or bind weakly (slightly positively charged), elute first while the analyte remains bound to the column.
This process is called cation-exchange chromatography.
The common types of stationary phases are:
- Quaternary amide for anion exchange.
- Suflonic acid for cation exchange.
Eluting Analytes in Ion-exchange Chromatography
How do you elute analytes in ion-exchange chromatography?
One way to elute the analytes is to increase the salt concentration of the mobile phase. These ions compete with the analytes for binding space on the stationary phase, which has a finite capacity.
Eventually, at a high enough salt concentration, the analytes are displaced and eluted.
Another approach is to change the pH of the mobile phase to alter the net charge of the analytes until they no longer bind to the stationary phase.
4. How Affinity Chromatography Works
Affinity chromatography separates molecules based on their ability to bind specific small molecules or biomolecules that are covalently linked to an inert polymer. Together these comprise the stationary phase
Think of affinity chromatography as a lock-and-key mechanism.
The small molecule or biomolecule on the stationary phase is the lock and your analyte is the key. The crude sample matrix is essentially a set of keys. But your analyte is the only key with the complementary shape (interaction) to fit the lock on the affinity medium.
Having your molecule bound to the stationary phase allows you to flush out the unwanted species. And when you’re ready, break the interaction to collect your analyte.
To apply this method, you must use a molecule with well-defined lock-and-key binding properties. Examples of specific binding are those found between an enzyme and substrate, antigen and antibody, and receptor and ligand.
Specific examples of affinity chromatography include:
- Polyhistidine binding to nickel ions;
- Maltose binding protein binding to dextrin;
- Glutathione S-transferase binding to glutathione;
- DNA binding to heparin;
- Biotin binding to avidin.
5. How Size-Exclusion Chromatography Works
Size-exclusion chromatography separates molecules by their size. Simple.
This method, also known as gel permeation chromatography or gel filtration, is unlike those described above because it exploits a physical, not a chemical, characteristic of the analyte molecules.
The stationary phase is a resin of porous beads that traps small molecules but not large ones. So, compounds with high molecular weights and large diameters are excluded because they’re too big to fit into the pores of the beads.
Crucially, the size of the pores in the beads is not one single value. It’s a distribution between two limits.
This distribution is what allows separation to occur since if all the holes in the beads were one size, analyte separation would be binary. (Yes, it does separate, or no, it doesn’t. No in between.)
Having trouble getting your head around it?
Another way to think about size exclusion chromatography is like this. Suppose you load a large polymer sample onto the column. During its time on the column, the polymer freely moves around without entering the bead pores because it is too large to do so.
Meanwhile, the small impurities constantly enter and exit the bead pores.
Consequently, the small impurities are retained on the column longer than the polymer analyte, which elutes first.
It’s like comparing someone driving on back roads with someone taking the highway.
The duration of travel through the column is longer for smaller analytes.
Chromatography Methods in Summary
So that’s how chromatography works. It’s all about exploiting molecular properties to force analytes to split their time between stationary and mobile phases.
If you can get the analyte to spend more or less time on the mobile or stationary phase, you can separate it from impurities.
Has this article cleared anything up for you? Have you used other column chromatography methods you want to let us know about? Please add to the list by commenting below!
Originally published August 2016. Reviewed and updated August 2022.
Further Reading
- Skoog DA, Holler FJ, and Crouch S (1997) Principles of Instrumental Analysis, 7th edition. Brooks Cole: Belmont