When I was a lab technician, I was often asked: “I need to use [chemical], but I can only find [chemical·6H2O], etc. Does it matter, and can I still use it?”
In many cases, the answer is no, it doesn’t matter, and yes, you can still use it.
Maybe you have asked yourself this question recently. Or perhaps someone has told you so but didn’t explain why.
Those water molecules are water of crystallization, and they seldom matter to your experiments.
Except, sometimes, they do. And they are a prime example of a broader phenomenon—solvent of crystallization—which will undoubtedly matter at some point.
So let’s explain everything you need to know about solvent and water of crystallization.
What is Water of Crystallization?
When a chemical crystallizes from a solution (say) during industrial preparation, it may cocrystallize with some solvent molecules. These solvent molecules become an integral part of the crystal structure of the chemical in question.
When the solvent is water, any extra molecules that cocrystallize with the chemical will be water.
This is called water of crystallization.
As mentioned, this water, when present, is integral to the crystal structure of the chemical in question.
The precise structure and arrangement of the water in the crystal structure don’t matter because we’re not solid-state scientists.
Three properties that do matter are:
- It exists.
- It occupies definite positions.
- It often helps hold the crystal together through hydrogen bonds.
Water of crystallization, whenever it is present, is there because it’s energetically and structurally favorable to include it when the crystal is forming. As I’ve said already, it’s integral. As much a part of the crystal as any other part. Check out Figure 1 below for an example.
It may incidentally impart desirable properties to a crystal, such as increased solubility, hardness, or conductivity.
For example, RhCl3 is insoluble in water, but RhCl3·3H2O is soluble. And anhydrous AlCl3 is a catalyst and strong Lewis acid, but hydrated forms are much weaker acids and unsuitable as a catalyst in organic chemistry.
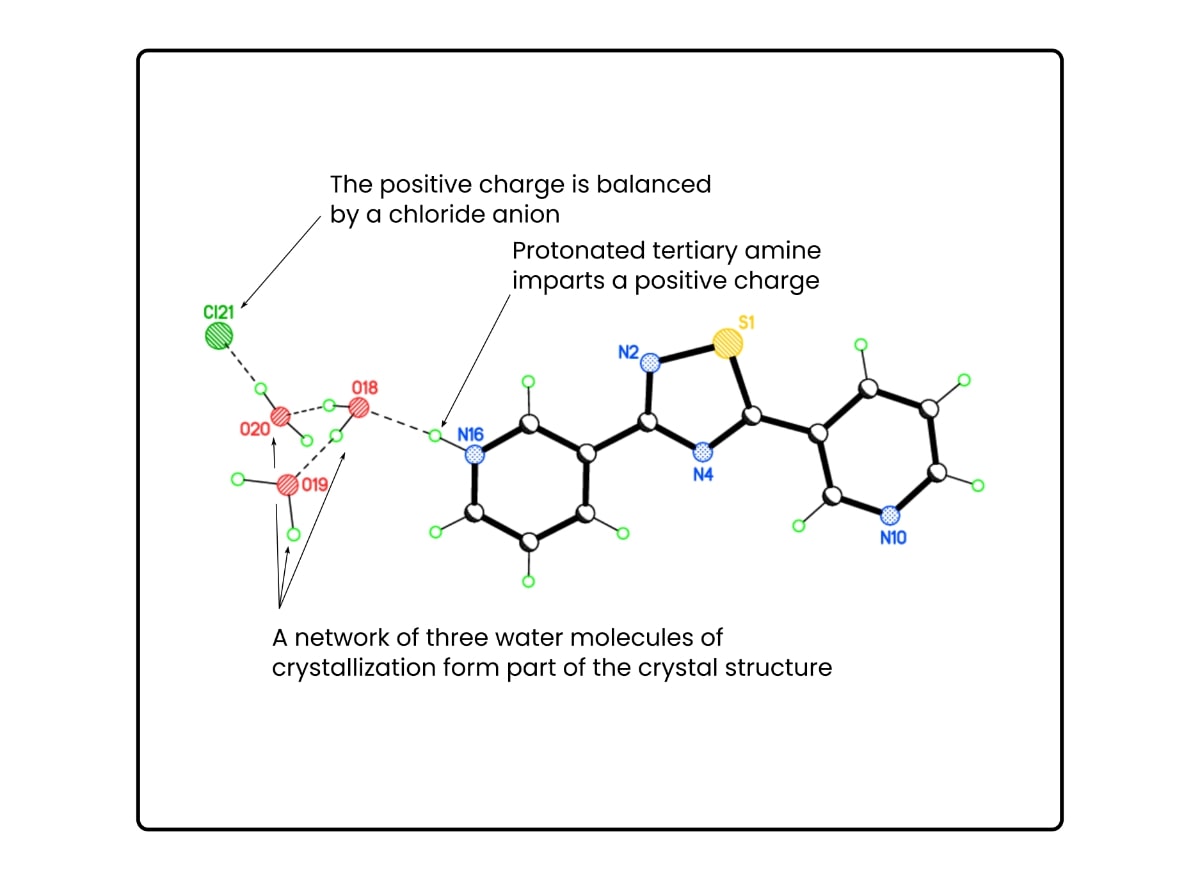
Note the dashed lines in the figure above represent hydrogen bonds.
There are three hydrogen-bonded waters of crystallization. One is hydrogen bonded directly to the main organic compound, and the two others are hydrogen bonded to it.
This 3:1 motif repeats for every molecule of organic compound in the crystal.
Note that in this example, the proton on the tertiary amine and chloride anion means there is an additional HCl molecule of crystallization.
Therefore, its formula would be something like this:
[main compound]H+·Cl–·3H2O
Or
[main compound]·HCl·3H2O
Both express the same thing. Starting to click?
Perhaps most crucially, it’s when the solvent of crystallization isn’t water, but another more intrusive solvent, that this information will benefit you. We’ll get to this later.
Common Examples of Water of Crystallization
Gypsum
Water of crystallization is responsible for the setting of Gypsum plaster (calcium sulfate or CaSO4).
We mix the dry gypsum with water, which sets as calcium sulfate dihydrate, CaSO4·2H2O.
Without the two molar equivalents of water, we wouldn’t get a wall or sculpture. Instead, we’d get something like the bag of fine powder we started with.
That’s to say, the water doesn’t all evaporate—some of it sticks around during the crystallization process and is, in part, necessary for the structural properties of plaster.
Proteins
Another fantastic example of water of crystallization is protein crystal structures. All living organisms on Earth are water-based. When their constituent proteins are crystallized to solve their structure, they drag some water molecules with them.
If they didn’t, they’d denature because this water of crystallization is part of their quaternary structure.
Note that phrasing it like this is the tail wagging the dog. It is more accurate to say that if the protein molecules in solution were totally dehydrated, they would denature. And denatured proteins do not crystallize because they are structurally heterogeneous.
Metal Hydrates
These represent chemicals that will probably make you ask the question at the top of the article.
Included are species such as iron(III) chloride dihydrate, magnesium(II) chloride hexahydrate, nickel(II) chloride hexahydrate, and anhydrous aluminum(III) chloride.
We use them extensively in biology. For example:
- Iron chloride is an additive in some cell growth media.
- Magnesium chloride is a PCR additive.
- Nickel salts charge metal-affinity columns.
- Anhydrous aluminum chloride is a catalyst in Friedel–Crafts acylation.
Plus, we mix them in various ways to impart desirable properties to our buffers and solutions.
Oh, and a bit of a random point. Anhydrous metal salts are usually quite hygroscopic (tend to attract moisture from the atmosphere). So take care to seal them properly after use. Otherwise, you won’t be able to accurately determine the concentration of any such reagent in your experiments because of the extra mass added by the water.
Can I Still Use it? Does Water of Crystallization Matter?
So, you’ve plucked a reagent off the shelf and noticed it has a different number of water molecules at the end of its formula than the one specified in your protocol.
Does it matter, and can you still use it?
In short: no, it doesn’t matter, and yes, you can still use it. Unless:
- You don’t intend to dissolve the reagent in water.
- The final percentage of water in your solution or experiment matters.
- You have to work under anhydrous conditions.
Why so?
Because the water of crystallization is labile (it won’t stick around) upon dissolution. If you dissolve the reagent into water, the water of crystallization will simply add to the bulk volume of water. So it won’t do any harm.
Note that I’m assuming that you usually intend to simply dissolve the reagent and crack on with an assy rather than (say) doing fancy pressure and conductivity measurements on the crystals themselves. Bitesize Bio is a biology blog, after all.
Just remember to weigh out the appropriate mass of the reagent so that your concentrations are accurate.
Every water molecule contributes 18 grams per mole of relative mass.
That means that water of crystallization sometimes contributes more (by mass) to the crystal than the species we are interested in and call the reagent by!
An Example Calculation
Let’s say a protocol asks you to prepare 500 mL of 0.5 M anhydrous magnesium chloride by dissolving 23.8 grams in 500 mL, but you only have the hexahydrate form in your lab.
How much should you weigh out?
The molecular weight of magnesium chloride is 95.2 g/mol.
The formula weight of water is 18 g/mol.
Thus, the formula weight of MgCl2·6H2O is
95.2 + (6 x 18) = 203 g/mol.
Dissolving 203 grams in a liter gives 1 L of a 1 M solution.
Dissolving 101.5 grams in 500 milliliters gives 500 mL of a 1 M solution. (Half the volume.)
Dissolving 50.8 grams in 500 milliliters gives 500 mL of a 0.5 M solution. (Half the molarity.)
So you would need to dissolve 50.8 grams in 500 mL.
Other Molecules of Crystallization
Molecules other than water can end up as solvents of crystallization. It depends on the solution from which a reagent is crystallized when it’s prepared.
Any species the reagent was initially solubilized with/in could get encapsulated in a crystal—if it’s sterically and energetically favorable to incorporate.
For example, acids (as we’ve seen), like hydrochloric and sulphuric acid, alcohols, ethers, and acetone, can cocrystallize with the primary reagent.
As can additives, such as sulfate, carbonate, and glycols.
So be aware!
Figure 2 below shows a good example of acetone of crystallization.
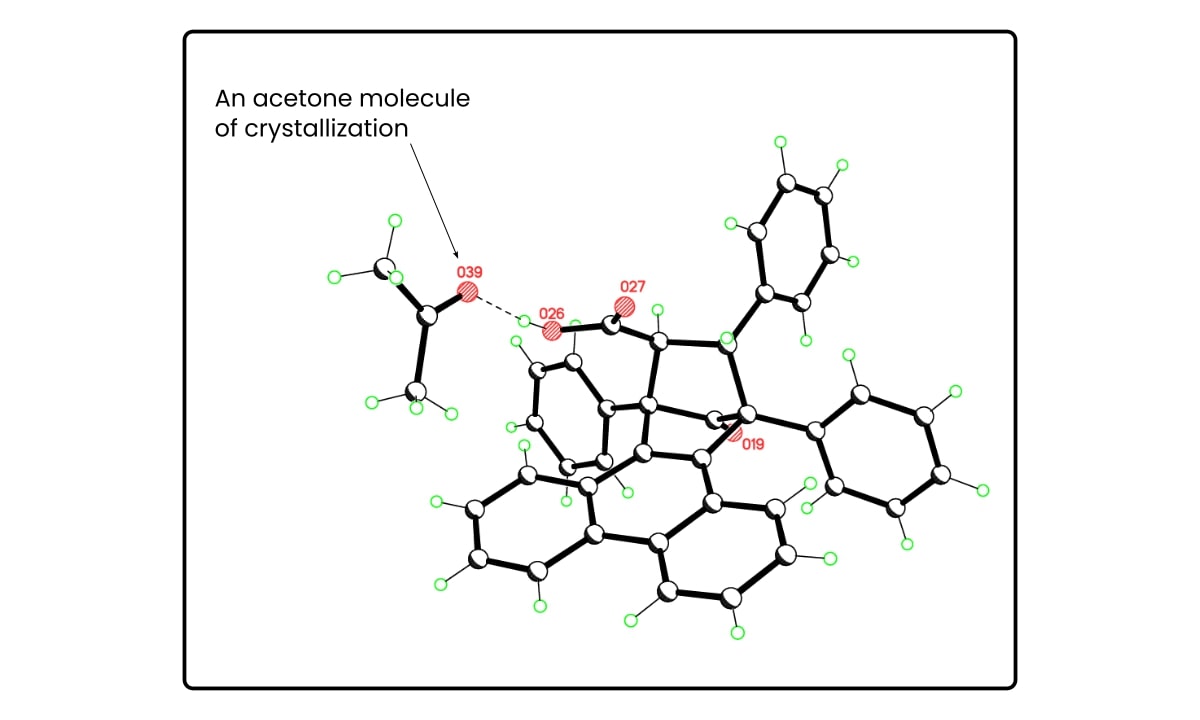
Crystals of this compound are 1:1 mixtures of acetone and the organic compound, probably because the compound was recrystallized from acetone.
Although you’ll never use this chemical, the fundamental point is that the solvent of crystallization exists in chemicals you will use, and it helps to understand what it is.
The good news is the solvent gets included in the reagent formula, usually found on its container.
It’s specified after an interpunct. (Also called a middle dot. Press alt+250 and thank me later.) For example.
reagent·2(solvent)
Ultimately, you have to decide, case by case, if any solvent of crystallization is going to impact your experiments. Or at least be aware of it to caveat your results or troubleshoot your experimental design if things go awry.
When Might Solvent of Crystallization Matter? Two Real-Life Examples
Medicinal chemists doing fancy organic syntheses to prepare lead compounds may need to work under anhydrous conditions occasionally. The classic example is the Grignard reaction to make new carbon-carbon bonds.
Here the halogenated reagents must be kept dry, or they break down.
Medicinal chemists do all sorts of fancy stuff, though—and more power to ’em.
For the rest of us, here are two prime examples of solvents of crystallization in the lab.
1. Tris·HCl
Tris·HCl (trizma®) is a version of tris base that’s often spoken of as pre-buffered tris because it has a lower pH than tris base when you dissolve it.
True, it does.
But more precisely, it’s tris base crystallized from hydrochloric acid. Some of the hydrochloric acid molecules come along for the ride. Crystals of it actually contain a 1:1 stoichiometric ratio of tris base and hydrochloric acid, as in Figure 1.
This matters for a few reasons.
First, tris base and tris HCl have different relative masses. So weighing out the same amount regardless of which form you’re using will result in a different final concentration.
Tris base has a relative mass of 121.1 grams per mole.
HCl has a relative mass of 36.5 grams per mole.
Therefore tris·HCl has a relative mass of 157.6 grams per mole!
Bear this in mind the next time you’re absent-mindedly following a recipe to prepare 10x SDS-PAGE running buffer.
Second, depending on the final desired pH of your solutions, you could accidentally add more salt to your experiments than intended.
If you dissolve tris HCl, and the pH is lower than required, and then you add some NaOH to increase the pH of your solution—you’re adding extra salt.
The Na+ comes from the NaOH, and the Cl– comes from the HCl.
In such cases, it’s better to use regular tris and not overshoot your intended pH value.
This leads me to an aside.
Neutralizing Solutions Generates Salt
Remember that whenever you neutralize HCl with NaOH or vice versa, you add salt to your experiment.
Want a real-life example?
Somebody converts chitin to chitosan via base hydrolysis at elevated temperatures and then neutralizes their experiment with HCl.
Powder X-ray diffraction then reveals whopping great sodium chloride peaks in their sample.
I’ve seen this. It’s not the end of the world because chitosan is insoluble, and you can dialyze the salt away.
It might be the end of the world if your enzyme assay or protein crystallization experiment is sensitive to the ionic strength of your solution.
If unaware of this phenomenon, you could struggle to recreate the conditions conducive to any initial success!
It might also impact any ion exchange chromatography you do. Especially if you initially extracted your analytes using strong acid or strong base.
Unfortunately, physical chemistry matters. So be aware of it.
2. TCEP·HCl
TCEP is a reducing agent that, unlike its alternatives, BME and DTT, does not readily hydrolyze in aqueous solutions.
Its stability makes it great for experiments that last several hours or days.
However, we purchase it as TCEP·HCl. So, for every molecule of TCEP in the crystal, there’s also a molecule of hydrochloric acid.
The hydrochloric acid of crystallization means that the pH of TCEP·HCl solutions is low.
Adding it directly to your experimental solutions could lower their pH and influence your results.
That’s why we should buffer TCEP·HCl solutions to a relevant pH using sodium phosphate or whatever buffer isn’t going to impact your experiments.
Removing Solvent of Crystallization
Do you desperately need to get rid of those water molecules or carbonate and so on? Here’s one thing you can try.
Heat your reagent up in a lab furnace as hot as you dare! Eventually, the solvent of crystallization should vaporize.
Just check that the reagent doesn’t do something dreadful at elevated temperatures, like exploding or producing toxic vapor.
No lead salts!
Note also that this could harm your reagent unless it’s some rock-hard mineral. It could also melt it or ruin its crystallinity. So think about if this matters.
And you probably won’t know whether or not the process has worked unless your reagent is a common one tabulated in the literature or you can replicate the conditions in a thermogravimetric analyzer.
Water (or Solvent) of Crystallization in Summary
You’re now equipped with the knowledge to decide if water (or solvent) of crystallization matters to your experiments.
When the solvent of crystallization is water, it won’t matter. When it’s something else, like acid or base, the pH of your solution will change. And that could make a huge difference.
This information might not come in handy today or tomorrow, but it will come in handy someday!
Have I missed something? Got something you want to add, or still a bit confused? Let me know in the comments section below.