Anyone who has performed mammalian cell culture will recognize the typical cell culture medium recipe: 1 bottle of DMEM, 10% serum, and a few other magical ingredients. Throw it together and put it on your cells to keep them alive and happy.
However, do you know what each ingredient does? Let’s look at the common ingredients in cell culture media and break down their roles.
The Six Main Ingredients in Cell Culture Medium
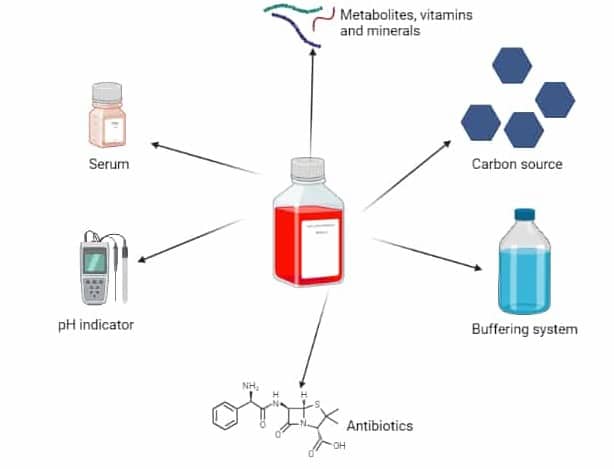
There are six main ingredients found in cell culture media (Figure 1):
- Carbon source (e.g., glucose).
- Buffering system (e.g., HEPES).
- pH Indicator (e.g., phenol red).
- Serum.
- Metabolites, vitamins, and minerals.
- Antibiotics.
Let’s dive deep into what each ingredient does and if they are strictly necessary for happy cells.
1. Carbon Source
This is the energy source for your cells. Every growth media requires a carbon source that cells can metabolize. This is often glucose, but it can also be other sugars such as galactose, hexose, fructose, or other carbon sources (e.g., pyruvate or glutamine).
Glucose is commonly added at a final concentration of 5.5 mM, which is approximately equal to the glucose levels found in human blood. However, you can buy media with wide ranges of glucose concentrations to suit your specific needs.
You can even buy glucose-free media to customize the concentration yourself to simulate high-sugar diabetic conditions or to study low-glucose stress responses.
2. Buffering System
Cells require a very specific pH from 7.2 to 7.4. If the pH moves out of this narrow range, cells cannot perform their usual cellular functions optimally to grow and survive. In incubators with high carbon dioxide levels, CO2 reacts with water to produce hydrogen ions that turn the media acidic.
To counteract the acidity generated by CO2 and maintain the optimal pH in culture, most media types use a chemical buffering system. However, some buffering methods interfere with cell growth.
The most common buffer system for mammalian cells with minimal biological impact is sodium bicarbonate. Sodium bicarbonate reacts with the hydrogen ions generated by CO2 and sequesters them to maintain pH. One disadvantage with sodium bicarbonate, however, is that it doesn’t buffer optimally at physiological pH. To get around this issue, researchers often use alternatives, such as HEPES, to increase the buffering capacity of certain media types.
3. Phenol Red
Because maintaining the correct pH is so critical to cell culture, many media formulations include a pH indicator called phenol red that turns yellow in acidic conditions (pH < 6.8) and pink in basic conditions (pH > 8.2). While generally not a problem, phenol red can mimic estrogen and, therefore, is commonly excluded when culturing cell types that express the estrogen receptor.
Media without phenol red is also available for colorimetric assays, such as the MTT proliferation assay, and for fluorescence experiments where the color spectrum of phenol would likely interfere with the readouts or image acquisition.
4. Serum
An essential component for many culture systems is serum, commonly fetal bovine serum—better known as FBS. Derived from blood, serum is an undefined mixture of sugar, salts, lipids, growth factors, and much more. Because of its biological source, it can be difficult to maintain reproducibility, as each batch of serum is unique. To circumvent this, some labs use chemically defined serum replacements, while others buy entire batches of one particular serum lot to ensure consistent results—at least until that lot runs out!
This variability, along with the nature of serum acquisition and the potential for contamination, has created debate over if serum should be used in culture media.
Some labs choose to culture cells in serum-free media, either using no replacement or synthetic alternatives that are more reliable depending on the needs of the cells.
5. Metabolites, Vitamins, and Minerals
If you look at the list of ingredients in DMEM, the majority fall into three main categories:
- Amino acids.
- Vitamins/cofactors.
- Inorganic salts (including calcium and magnesium).
Many of these components are common in all cell culture media, and the exact combination and concentrations have been optimized for cell growth. Despite this, many labs supplement their media with additional non-essential amino acids to prevent cells from using glucose or glutamine for their synthesis. Additionally, some companies produce magnesium and calcium-free media for specific studies, so check the components carefully when you order!
6. Antibiotics
Many labs include antibiotics in their cell cultures to prevent unwanted bacterial or fungal growth, even when practicing aseptic technique. The most popular choice of antibacterial supplement is a combination of penicillin and streptomycin (pen/strep) at a final concentration of 50–100 IU/mL penicillin and 50-100 µg/mL streptomycin in complete cell culture.
However, the use of antibiotics in cell culture media is a topic for debate.
Should We Use Antibiotics in Cell Culture Media?
Some labs choose not to use antibiotics as they can:
- Have unwanted effects on your cells, including significantly altering gene expression. [1]
- Can mask low levels of bacterial contamination.
Depending on your needs, you can decide whether or not to include them in your media.
There is also the suggestion that they are unnecessary if you maintain good culture hygiene practices, and reliance on antibiotics can make researchers lax on this.
Common Type of Cell Culture Media
So now you know what is in most cell culture media, let’s discuss some of the most commonly used media. Table 1 summarizes some of the most popular mammalian cell culture media available.
Media | Features |
EMEM (Eagle’s minimal essential medium) | A minimal medium, first developed in 1959. Useful as a base for modification for specific requirements |
DMEM (Dulbecco’s Modified Eagle’s Medium) | Similar to EMEM but with higher concentrations of vitamins and amino acids |
RPMI-1640 (Roswell Park Memorial Institute -1640) | A more complex medium commonly used for human lymphocytes. Can support a wide range of cell types |
IMDM (Iscove′s Modified Dulbecco′s Medium) | A modified version of DMEM. Useful for high-density cultures |
McCoy’s 5A | A modification of Basal Medium 5A. Used in the culture of many primary cells and explants from biopsies |
Ham’s F-12 | Commonly used for serum-free growth of Chinese Hamster Ovary (CHO) cells. Also used for serum-free growth of other mammalian cells |
Note that often the above media is modified in some way by suppliers, and the composition of the medium may vary between suppliers. Carefully check that the medium you are purchasing fits the needs of your cells and is the expected composition. Changes to the cell culture medium used could affect the growth and behavior of your cultures.
Which Medium Should I Choose?
There is no straightforward answer to this question, as it depends on many factors, including the cell type you are culturing and the experiments you are performing.
The best place to start is to look at the cell line you are using and refer to the supplier’s instructions, as these will usually include details of the recommended medium. For specific applications, the literature is your friend.
The most important point is to understand why you are using that particular medium (e.g., because it’s low glucose, phenol-free, or formulated for fibroblasts).
Cell Culture Media Summarized
There are six main components in cell culture media that are added to keep your mammalian cell lines happy and healthy. Different media types vary in ingredients and amount, making them suitable for varying applications. While the use of serum and antibiotics in cell culture medium is not uncommon, there is a debate about the potential pitfalls of including these in cell culture medium.
Before you choose or use a cell culture medium, read the ingredient list and know why you are using that particular composition. You may find that other media suits your cell line or application better.
Happy Culturing!
References
- Ryu, A.H., et al. Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci Rep 7, 7533 (2017).
Originally published in October 2017. Reviewed and updated, October 2022.