Have you found a paper that uses gas chromatography to analyze a sample that is similar to yours, and now you are tempted to try it too?
But you’re hesitant because you don’t know much (or anything!) about gas chromatography.
Or maybe the paper’s results just don’t make any sense without a working understanding of it.
Good news—this article will help you.
Stick around as we explain the essential principles, function, and instrumentation of gas chromatography.
What Is Gas Chromatography?
You may already know that chromatography covers a wide range of analytical techniques that are used to separate, identify, and quantify compounds. If you are familiar with other chromatography methods, such as HPLC or GPC, then you already know more than you think!
In gas chromatography (GC), compounds are analyzed, unsurprisingly, in the gas phase!
This doesn’t mean that your sample has to be a gas at ambient conditions, although it can be.
If not, the gas chromatograph (the instrument) will vaporize your sample for you. After this, it will take a steamy journey through a column, where it partitions between a mobile phase and a stationary phase as per all other analytical techniques.
The precise extent of this partitioning dictates how much time any sample spends on the column and, therefore, the time it takes to reach the detector.
This measurement is called the retention time, tR. It is determined by the detector when a compound exits the column. In strict terms, we call this process of exiting the column and subsequent detection “eluting.”
How Does Gas Chromatography Work?
We already mentioned that gas chromatography analyzes compounds in the gas phase.
Because the injector port is contained in an oven (see Figure 1 below), your sample will be vaporized immediately after injection.
The gaseous molecules are then mixed with the mobile phase. This is also known as the carrier gas because it does exactly that: it carries your sample through the column.
If your sample is a mixture of compounds, they will separate as they travel at different speeds through the column.
This happens because while a compound travels through the column with the mobile phase, it spends some time sticking to the stationary phase that is bonded to the column.
The key point is that different compounds spend different lengths of time stuck on the stationary phase.
The exact time that a compound spends interacting with the stationary phase will depend on its chemical composition (and the chemical composition of the stationary phase).
So, when a sample containing a mixture of chemicals is injected into the chromatograph, they separate out and exhibit a characteristic tR.
If you need to think of it another way, you can imagine that a compound “held up” by the stationary phase will have a longer tR than another traveling freely with the mobile phase.
And all compounds are held up to a greater or lesser extent.
GC Components: A Closer Look
Figure 1 shows the basic components that make up a GC system.
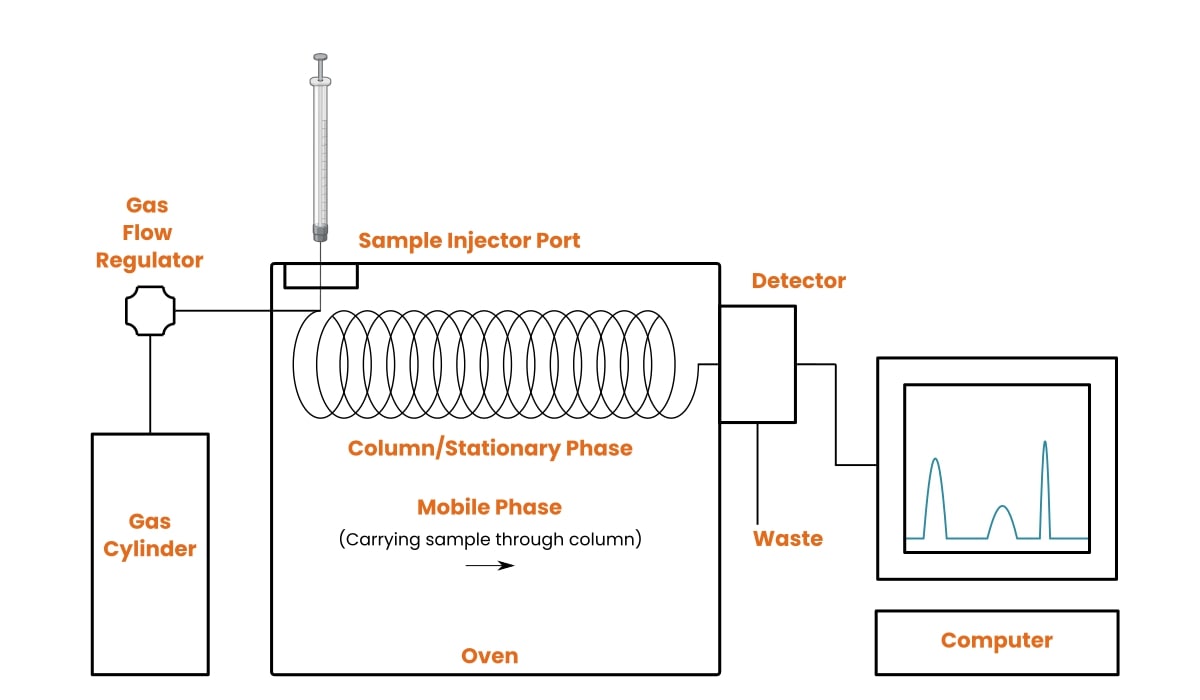
1. Sample Injector
The type of sample may vary greatly depending on the study’s aims.
Typically, GC samples consist of non-polar, low molecular weight, thermally stable, and vaporizable chemical material.
Samples may be a single substance, a mixture, a liquid, or a solid that is dissolved in a solvent. And only a few microliters need to be injected into the GC for analysis. This is done using a syringe.
It is also possible to analyze gaseous samples such as air and breath. In these instances, injection is usually performed through a gas valve using a gastight syringe.
2. Column/Stationary Phase
This is where the separation of sample compounds takes place.
The column is a coiled tube made of metal or glass material that can withstand high temperatures. Columns vary in both length and diameter.
Typical dimensions are a diameter of 3 mm and a length of 5 m.
Bonded to the inside of the column is the stationary phase. This can be several different materials with varying polarities. It may also be either solid or liquid. In any case, the stationary phase interacts with your samples to achieve separation.
The stationary phase is assembled either as a compacted material (packed column) or as a wall-coating film (capillary column).
3. Mobile Phase
The carrier gas is introduced from a gas cylinder into the gas chromatograph. It moves through the column at a constant flow rate and exits at the detector outlet.
Unlike some other analytical methods, the mobile phase in GC does not interact with chemicals and only serves to carry them. Because of this, the carrier gas must be inert. Examples include helium, nitrogen, and argon.
The type of detector on the GC usually determines which gas is used. It is not something that you would have to decide since a working GC will already have a gas cylinder connected to it.
4. Oven
Like any other oven, a GC oven provides heat. But instead of baking goods, this oven gives you vaporized material right after injection. Additionally, it keeps the column heated so that you continue to have gaseous molecules traveling through.
Its temperature could vary from room temperature to 300°C, though the limits vary by instrument. A temperature program can be programmed electronically to maintain a constant temperature or gradually increase (ramping).
The program that you select will depend on the nature of the sample and your study aims.
5. Detector
This device at the end of the column senses each compound as it elutes.
The data recorded by the detector is transmitted to a computer that produces a two-dimensional plot called a chromatogram.
There are many types of detectors with varying detection methods and limits. They may also be combined. A particularly powerful technique arises when a GC is coupled with a mass spectrometer (MS).
A GC coupled with an MS is known as a GC/MS system. It produces a mass spectrum for each sample component and the GC chromatogram.
How to Read a Gas Chromatogram
As mentioned, regardless of detector type, gas chromatography experiments produce a chromatogram.
A GC chromatogram (Figure 2) is a visual output of the data recorded by the detector. It is presented as a plot of detector response (y-axis) versus retention time (x-axis).
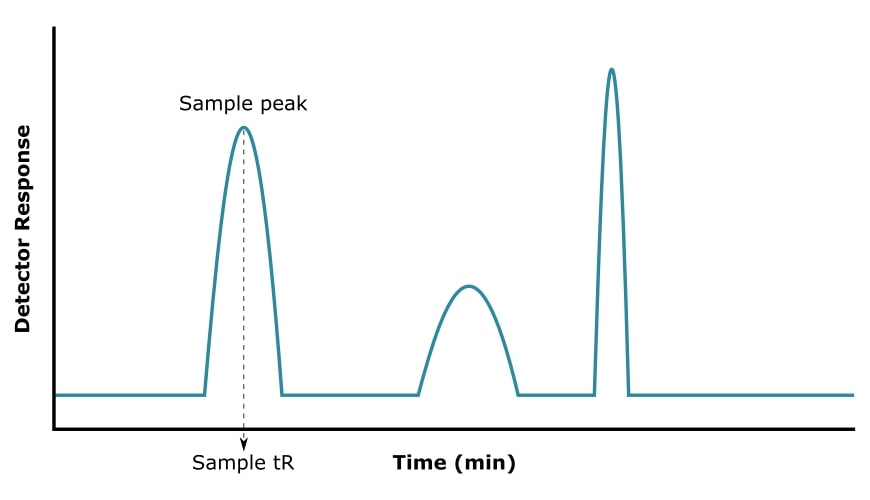
The chromatogram tells you several different things. I will break it down into three main categories.
1. The Nature of the Sample
To evaluate the complexity of your sample, you can count the number of peaks. Each compound detected by GC should appear as a single peak with a characteristic tR.
If you injected a mixture and the chromatogram shows three peaks, then this tells you that your sample contained three different compounds.
But let’s say that you wanted to confirm the purity of a sample. In this case, you expect a single peak, and hopefully, that’s what you get!
2. The Identity of the Sample
A substance can be identified by matching its tR with a literature value or by injecting reference material. The catch with this is that you have to use the same conditions for each injection.
This is important because the tR depends on factors other than the compound identity. These include carrier gas flow rate, temperature, and column length. The GC/MS systems are the most powerful for compound identification as they enable identification by mass.
3. The Amount of Sample
The peak area on a chromatogram is proportional to the concentration of the corresponding sample. Modern GC software will integrate every peak and provide this information to you.
The relative amounts of compounds in a sample can be determined by comparing the peak areas, or you can calculate the actual concentrations by using a standard calibration curve.
Carrying You Through Gas Chromatography
Hopefully, this article has given you a solid understanding of the principles and instrumentation involved in GC.
It can be an intimidating technique at first, but after giving it a couple of tries, you will get the hang of it!
We’ve also touched on sample type and compatibility, which should help you decide whether or not gas chromatography is for you.
I recommend watching this video for a visual tutorial on GC and reading about some aspects of GS in more detail if you want to expand your GC knowledge.
Good luck!
Originally published July 2016. Reviewed and updated October 2022.