Your advisor tells you that he wants you to use HPLC to analyze your compound. You know that you’ve heard of this technique before, but you can’t remember what HPLC stands for, let alone how to go about doing it! We’ve all been there, though.
Fear not! In this article, we will remind you about the power of the high performance liquid chromatography (HPLC) column. we will take you through the principle behind HPLC and remind you of its uses. You will be ready for the lab in no time!
How Does High Performance Liquid Chromatography (HPLC) Work?
High-performance liquid chromatography, or HPLC, is a long name for a powerful technique based on the simple fact that individual compounds behave differently in water.
HPLC separates and purifies compounds according to their polarity, or their tendency to like or dislike water. To put polarity into context, oil is an apolar liquid that doesn’t mix with water. Ethanol, on the other hand, is polar and mixes very well with water.
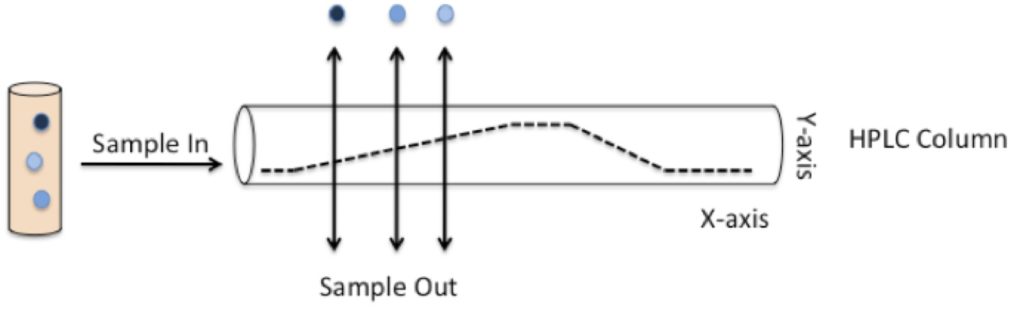
A simplified HPLC process is shown in Figure 1 below. First, let’s look at the main components involved in HPLC and demystify some unavoidable jargon!
The Components of the HPLC Process
The HPLC column
The column is the workhorse of the HPLC machine. The resin in the column, also known as the stationary phase, is often made of silica but can be composed of a variety of substances. The column itself is very compact. Long carbon chains, which are apolar, functionalize the silica particles. The longer the chain, the more apolar the column. Researchers commonly use columns containing 18-carbon chains, known as C18 columns.
The HPLC Sample
Sample types vary greatly depending on the field and the type of compounds in question. HPLC can be used to analyze compounds in biological specimens (urine, blood, saliva, and muscle), environmental samples, medicinal chemistry (drugs), and microbiology (toxins produced by fungi and bacteria).
Injection of Sample
Samples are injected into the HPLC column. Injection used to be carried out manually, meaning that some poor soul had to sit by the HPLC machine for hours on end injecting each sample with a syringe—sometimes all night long!
Luckily, newer models have an automatic injector, reducing the manual input and allowing higher throughput. Software equipping modern machines allow the user to input a list of samples, the amount of each, and in what order the samples should be injected. So, you can enjoy your lunch while your HPLC runs itself!
The Mobile Phase
The mobile phase is really just a mixture of water and an organic solvent (usually acetonitrile or methanol). The mobile phase gets its name from moving through the column (it’s mobile) and simultaneously elutes (or flushes out) compound from the column.
Compounds are often eluted along a concentration gradient. The term “concentration gradient” sounds complicated, but it just means that the percentage of water in the mobile phase decreases over time, while the percentage of the apolar solvent increases simultaneously. So, the mobile phase gradually becomes more apolar.
The HPLC run
HPLC can be performed in a number of modes. The most commonly used method is known as reversed-phase (RP-HPLC) that is described in this article. In this mode, the HPLC machine separates compounds starting with the most polar and ending with the apolar compounds. For all modes, a high-powered pump moves the sample and the mobile phase through the column. A typical run can take between 10-60 minutes.
The Principle Behind HPLC: A Closer Look
Now that you have an idea of the components involved, let’s move on to the principle in a little more detail.
The HPLC separates compounds based on polarity. But how does this actually work?
As the concentration gradient kicks in, the solvent concentration increases while the water concentration decreases. The increase in solvent concentration causes the mobile phase to become more and more apolar. Compounds contained in the sample will stick to the carbon chains in the column, with the most apolar compounds sticking the strongest and the most polar compounds sticking weakly if at all.
Figure 1 shows what happens to a sample containing a mixture of compounds after injection into the column. The compounds bind to the column and are eluted out at different times, depending on their polarity. The time that each compound elutes from the column is known as that compound’s retention time (Rf).
Understanding the HPLC Output
The output or results of an HPLC run is usually viewed as a chromatogram (Figure 2). The horizontal series of peaks seen in the chromatogram represent compounds eluted from the column with different Rf values. Modern HPLC equipment is often coupled to a diode array detector (DAD), allowing the user to look at the resulting chromatogram of separated compounds in wavelengths from 190 nm to 900 nm. If the compounds under investigation are known, the user can choose to look only at one or a few selected wavelengths. For instance, cocaine can be observed at 254 nm.

Applications of HPLC
Within biology and medicine, HPLC is often used as an analytical tool to assay biological and environmental samples for the presence or absence of known compounds (for example, metabolites, drugs, toxins, pesticides) and can assist in the identification of unknown compounds.
Within chemistry, however, HPLC is routinely used to monitor chemical reactions and to determine the purity of the products. In addition, the process of HPLC can be modified to preparative HPLC, whereby compounds of interest can be purified for further use.
HPLC can come across as being very complicated, but rest assured—just like most other lab techniques, it makes a lot more sense when you actually do it.
Originally published in 2014, republished in 2016.