Does your western blot look like someone sat on black playdough? If you’re lamenting over yet another blotchy or bad western blot, don’t fret. The cause can often be a poor transfer, which can easily be fixed by optimizing your western blot transfers.
Here are three tips to optimize your Western blot transfers to enable you to get clear blots consistently.
What Are the Parts of the Transfer Stack?
Before discussing ways to optimize your western blot transfer, it’s worth familiarizing yourself with the parts of the transfer stack. Check out the simple illustration in Figure 1 below.
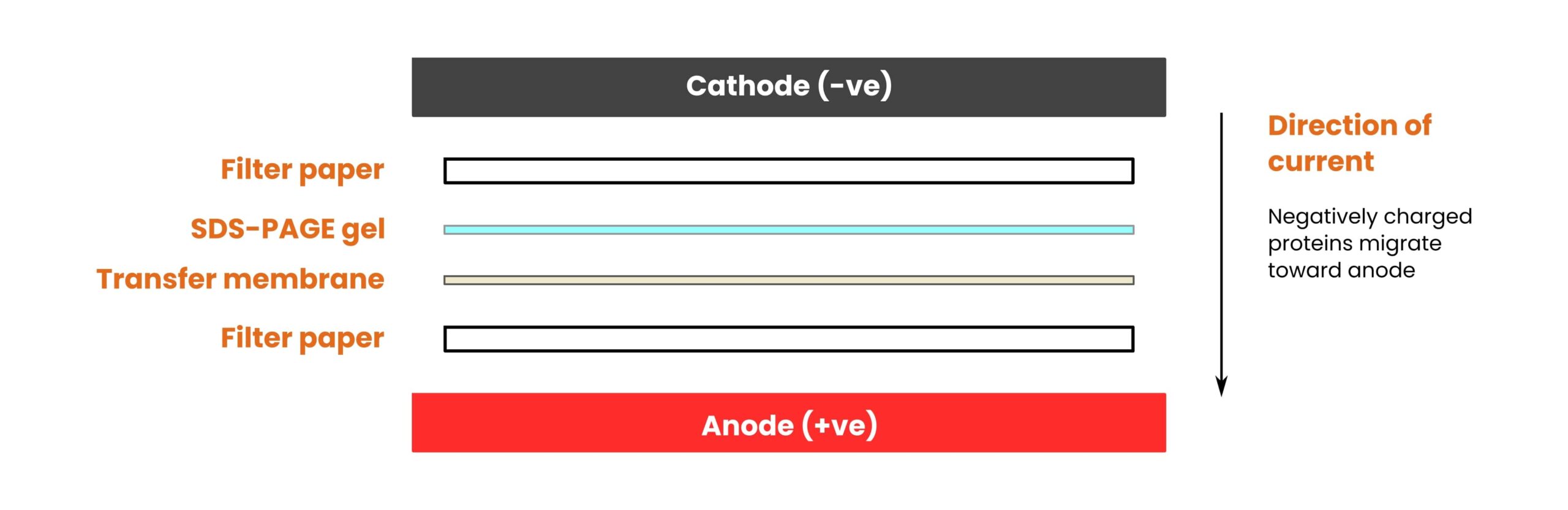
Knowing the pieces of the stack will also make you a better scientist, which is never a bad thing!
This is because the stack itself can result in a poor transfer. When, for example, the transfer device becomes encrusted with buffer salts. Wipe it down with damp tissue paper if you notice this is the case.
Going from top to bottom, the pieces are as follows:
- The cathode (negatively charged).
- A filter paper.
- Your pre-run SDS-PAGE gel.
- The transfer membrane.
- Another filter paper.
- The anode (positively charged).
Two other key points are worth mentioning here. Membrane choice and transfer buffer.
Membrane Choice
Usually, you have a choice between two transfer membranes: nitrocellulose membranes and polyvinylidene fluoride (PVDF) membranes.
Choosing between them comes down to a choice between binding capacity vs. background. PVDF membranes can bind more protein than nitrocellulose membranes but may yield higher background.
You also get a choice of pore sizes, so make sure to pick one suitable for your protein size. While 0.45 µm is sufficient for most proteins, a smaller pore size might be necessary if you work with smaller proteins.
Be sure to check out this article that explains the differences between the two membranes in detail.
Transfer Buffer
Depending on the type of transfer you are performing, the transfer stack is usually wetted with a transfer buffer.
You probably have a standard recipe for this somewhere in your lab. But be open to the idea that this buffer might not be suited to your samples.
Designing a tailor-made transfer buffer can get quite technical. Fortunately, Bio-Rad has an article discussing transfer buffers, their composition, and compatibility.
Now let’s take a look at the different types of transfers in Western blotting.
What Are the Different Ways of Transfer in Western Blotting?
There’s already an excellent article on Bitesize Bio that discusses the different ways of transfer in Western blotting. So, I’ll just give you the crib notes.
There are three main ways of transferring proteins in Western blotting:
- Standard wet transfer method.
- Semi-dry/rapid transfer method.
- Capillary transfer method.
The wet transfer method is used in most laboratories and is probably the one you use. You manually soak the stack in a transfer buffer (hence wet) and assemble it yourself into a transfer sandwich.
The semi-dry/rapid transfer involves purchasing pre-wetted transfer sandwich components and using this with proprietary transfer devices such as the iBlot™. Because of this, it’s more expensive than wet transfer.
Capillary transfer relies on diffusion to move your samples from the SDS-PAGE gel onto the blotting membrane instead of an electric current. Because the apparatus for wet transfer is now relatively cheap, the capillary transfer method is somewhat archaic.
How Long Should I Transfer My Western Blot For?
Transfer times are empirical and based on the properties of your samples. For example, molecular weight and hydrophobicity influence protein retention.
This means there is no easy way to determine how long you need to run the transfer to completely transfer all your proteins.
Instead, you will need to optimize the transfer time and conditions based on your equipment and the nature of your sample.
But how will you know when your transfer is complete? Well, this brings us to our transfer tips since these will enable you to optimize your Western blot transfers to achieve near-complete transfer.
Let’s get into them.
3 Tips to Optimize Your Western Blot Transfer
1. Use a Pre-Stained Molecular Weight Ladder
Use a pre-stained protein ladder to track the transfer of proteins from a gel to a membrane. Your sample proteins on the SDS-PAGE gel will still be invisible, but you can check if the brightly colored ladder bands are still on the gel or have fully transferred to the membrane.
Note that proteins of different molecular weights will migrate at different speeds—there is no magic moment at which all the proteins jump from the gel to the membrane.
Instead, aim for a point at which most of the larger proteins have migrated to the membrane, and most small protein material is still on the membrane—not through it.
Using a ladder in which each band is a different color can be beneficial. It will enable you to keep track of the transfer efficiency of differently-sized proteins.
Remember, if you’ve opened your transfer cassette to “peek” at the ladder, this will introduce air bubbles to the transfer stack. So you probably want to use this technique to optimize transfer times—not when you’re running critical samples.
2. Stain Your SDS-PAGE Gel with Coomassie Blue
But only after the transfer step! Let me explain.
Another simple way to monitor the efficiency of protein transfer is to stain the gel with Coomassie after your transfer is complete.
It’s a quick and easy procedure that will give you a good idea of how much protein is still in the gel.
If the gel is almost entirely blank, then your transfer was successful.
But if most of your gel shows blue sample bands, your transfer needs to run longer.
Again, this is a post hoc check for transfer efficiency. Once you’ve stained the gel, you cannot set it up for a second transfer. Instead, you’ll have to run a second gel and do the transfer again using the adjusted conditions.
So remember to do this test before you run your precious samples!
3. Transfer Your Gel onto Two Membranes
The two methods described above are excellent ways to determine if the transfer duration was too short.
To test if your transfer is too long, insert two transfer membranes into your transfer stack, one right behind the other.
Then, go ahead and blot both membranes as you normally would.
Just be sure to label which was farthest away from the gel!
If your transfer conditions are good, most of your protein will be on the membrane closest to the gel.
If your transfer is too long, you will detect protein on the membrane that is farthest from the gel.
This is more likely to occur to the small proteins first so pay particular attention to where the lower molecular weight ladder bands should appear.
You can also try staining the second membrane with Coomassie blue (or something more sensitive). This should show you all the proteins transferred to that layer, not just your protein of interest.
Did These Tips Work for You?
We’ve covered three simple tips you can use to optimize your Western blot transfers. Hopefully, this improves the quality and clarity of your blots, making them easier to interpret and freeing you up to get on with more pressing research problems.
Did these tips help you out? Have you got any of your wisdom to share? Let us know in the comments section below!
Want all your Western blot buffer recipes, an ECL reagent recipe, and an easy protocol all in one place and on hand? Download our free Western blot cheat sheet.
And for more information on these transfer controls, check out this Western blot troubleshooting video from Agrisera.
Originally published August 2011. Reviewed and rewritten in November 2022 using previously created articles from Emily Crow and Joanna Porankiewicz-Asplund.






