If you’ve worked in a lab, odds are you’ve had an encounter with filtration of one sort or another. Do you understand exactly how filters work, though? Or have you wondered why certain filters are used for certain lab applications?
Although filtration probably isn’t the primary focus of your projects, having a basic understanding of filtration and how membrane filters work is important because most researchers rely on filters at some point or another.
This article will review how filtration works and look at the different types of filters and considerations for their use so that you’ll have that baseline knowledge when considering filtration for numerous applications.
What is Filtration?
Let’s start by defining what exactly filtration is. Simply defined, filtration is the process of separating or removing solids from fluids. While that may sound straightforward, consider the huge diversity of new and complex technologies and materials and the dynamics that occur at macroscopic and microscopic levels during filtration! Indeed, filtration is a field of study that has existed for millennia to improve drinking water quality.
Note that filtration is not like adsorption, in which particles in a solution bind to a matrix based on charge or affinity to become trapped. Nor is it sieving, which uses a single porous layer to prevent particles larger than the layer perforations from passing through.
Uses of Filtration in the Lab
Besides providing an efficient and effective means of sterilization when working with liquids that can’t be autoclaved, filters can remove particulate matter prior to processing on analytical platforms like high-performance liquid chromatography (HPLC) and mass spectrometry (MS).
Filters are also used when purifying and concentrating macromolecules. For instance, gel filtration chromatography is capable of separating large peptides and proteins from one another using porous beads that allow larger molecules to pass through the matrix (bypassing the pores), while simultaneously slowing down smaller molecules that travel through the pores.
How Filtration Works
To discover what happens when fluids are filtered, let’s first classify filters into their three main categories:
- Depth filters.
- Membrane filters.
- Porous gel matrices.
Depth Filters
Depth filters are made of fibrous media and do not have defined pore size or structure. Particles passing through depth filters become entrapped or adsorbed due to the tortuous path they must travel to exit the filter (Figure 1).
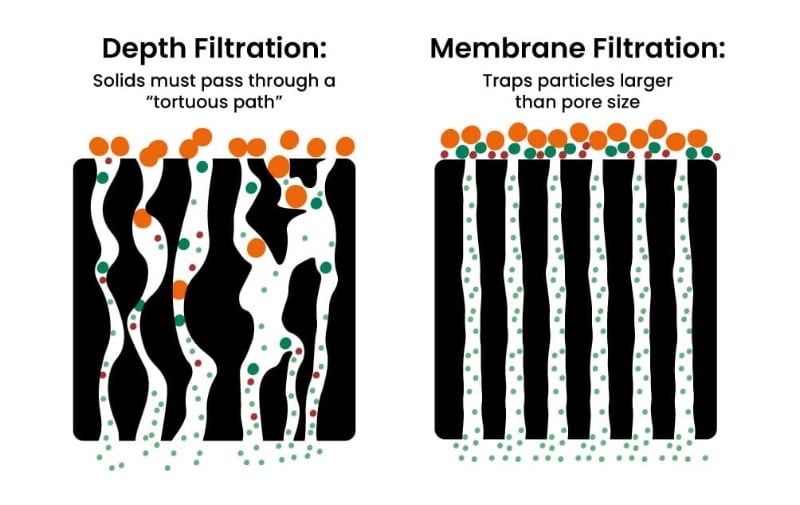
Depth filters are commonly used in water purification processes because they are relatively inexpensive and the total removal of particles below specific sizes is not strictly required.
Membrane Filters
In contrast to depth filters, membrane filters have a defined pore size and structure and more stringently prevent particles above a certain size from passing through the material (when used properly, that is!). Because we typically want to remove 100% of particles above a specific size in the lab, we typically use membrane filters, so they’ll form the focus of this article.
Gel Filtration
Finally, gel filtration (also known as size exclusion chromatography, molecular sieve chromatography, or gel permeation chromatography) relies on porous beads that are packed into a column. When a sample containing macromolecules of multiple sizes is applied to the column, the larger molecules bypass the beads because they are excluded from the pores, but smaller molecules are slowed down by their travel through the pores.
While depth filtration plays a critical role in economic and large-scale filtration processes and gel filtration has excellent uses in analytical and purification techniques, the rest of this article will focus on membrane filters, which are intended to completely remove particles above a certain size.
Membrane Filtration and Particle Size
The number one characteristic to understand in filtration is the size of the filtered particle (Figure 2).
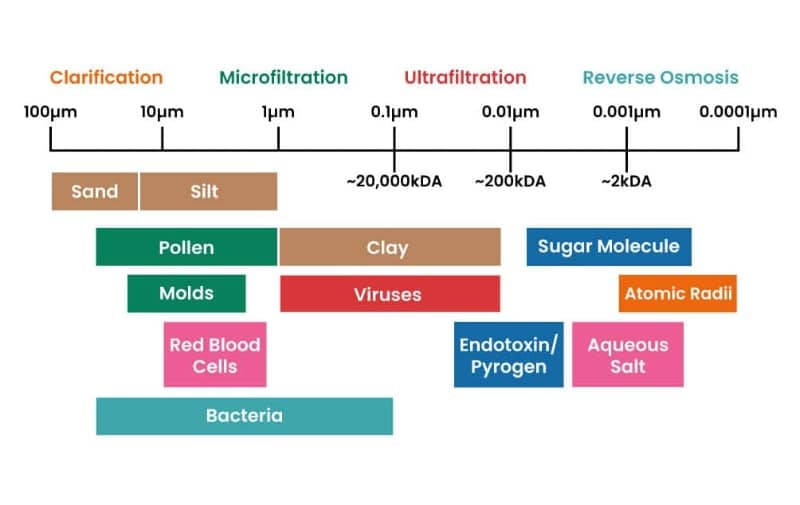
After all, removing macroparticles like visible precipitates from a solution is very different from separating out microorganisms. From largest to smallest particle filtered, membrane filters can be further divided into four categories. [1]
1. Clarification
Often used to “pre-filter” or “clean up” samples before using more stringent filters, clarification filters remove relatively large particles and debris. They are typically rated for removing particles larger than 5 µm—about 10 times smaller than the smallest particle one can see with the naked eye.
Clarification is an important part of sample preparation when you’re planning to run liquids on analytical equipment like HPLC or gas chromatography. If you are purifying proteins on a gel purification column and see visible debris or aggregates, clarification is an absolute must to prolong the life of your expensive columns as well!
2. Microfiltration
When you’re looking to remove microbes, including bacteria and yeasts, microfiltration is the way to go. Ranging from 0.035 to 10 µm, some of these filters can capture bacteria as small as the aptly named Brevundimonas diminuta, which has a cell diameter of only 0.3 µm!
The most common application of microfiltration is sterilization. Not all components in solution can be autoclaved. Given that several vitamins and antibiotics are heat sensitive and cannot tolerate hot sterilization processes, microfiltration offers an easy way to remove contaminating organisms while preventing degradation of these molecules. Sterilization filters are rated for removing particles of >0.2 µm and are often tested with B. diminuta to verify their efficacy.
3. Ultrafiltration
When working with even smaller particles, scientists use ultrafiltration techniques. Here we find filters that can concentrate and separate macromolecules, removing those molecules that are smaller than sizes rated by the filters.
Ultrafiltration is commonly used in protein purification and concentration, where centrifugal membrane and cassette filters are convenient tools to clean and isolate your protein of interest. Don’t be put off if you hear them called “semi-permeable” filters—this simply means that proteins below the size rated on the filter pass through, while proteins above the size remain in a resulting concentrate. Ultrafiltration membrane filters range from 0.001 to 0.05 µm (or approximately 1–1000 kDa in protein-speak) and require pressure-driven processes such as centrifuge or vacuum to work.
4. Reverse Osmosis
A reverse osmosis (RO) system separates ions by overcoming osmotic pressure, forcing a solvent to move from a high solute concentration to a low one. You may use “RO” water in your lab from time to time, especially if you need water free of ions such as sodium chloride that are typically found in much higher concentrations in non-filtered water. [2] This method of filtration is effective for particles as small as <100 Da!
Selecting and Using Membrane Filters in the Lab
Regardless of the particle size, you aim to remove from a solution (or concentrate, in the case of certain protein purification filters), there are some general considerations when selecting a membrane filter.
Choosing the wrong membrane filter for your application can lead to some unintended consequences, including failure to filter in a reasonable time, leaching chemicals into the filtrate, or simply not removing the right particles from your solution.
Perhaps the most dangerous mistake is using a solvent that dissolves your membrane filter, leading to no filtration at all! You should always carefully consider sample and filter compatibility when making filtration decisions.
Table 1 provides an overview of some of the most common filters for various applications, but there are an enormous variety of filters available within each material class. Be sure to check out your preferred vendor’s website for even more options.
Table 1. Overview of common filters.
| Membrane Filter Material | Pore Sizes (µm) | Hydrophobicity | Key Properties | Suggested Applications |
| Glass Fibers (Borosilicate or Quartz) | 0.2–0.8 | Hydrophilic | • Low cost • Compatible with aqueous solvents | Prefiltration/clarification |
| Polypropylene | 0.6–80 | Hydrophobic | • Large pore size • Good solvent resistance | Prefiltration/clarification |
| Nylon | 10–180 | Hydrophilic | • General purpose filter • Uniform pore structure • Large pore size options | Prefiltration/clarification |
| Cellulose Esters and Acetates | 0.025–8 | Hydrophilic | • Versatile materials • High protein binding | Sterilization, general clarification |
| Polytetrafluoroethylene (PTFE) | 0.45 | Both hydrophilic and hydrophobic options exist | • Fast-flowing • Compatible with a broad range of solvents • Minimal extractables | HPLC mobile phase filtration, clarification |
| Polyvinylidene fluoride (PVDF) | 0.1–5 | Both hydrophilic and hydrophobic options exist | • Durable • Low protein binding* • Solvent resistant | Gas filtration and venting, solvent filtration |
| Polyethersulfone (PES) | 0.22, 0.45 | Hydrophilic | • Fast-flowing • High throughput • Low protein binding* | Sterilization of biological solutions |
| Polycarbonate | 0.5–250 | Hydrophilic | • Tight pore size distribution | Collection of particles on surfaces for analyses, air monitoring, microscopy |
The number one priority when filtering is to ensure that your sample is chemically compatible with the filter membrane; that is, you need to ensure your sample won’t be altered by the membrane filter or affect the filter’s integrity. Many companies provide a chemical compatibility chart to cross-reference when choosing a filter. Strong acids and bases can dissolve certain filters, so double-check the vendor’s recommendations and datasheets for your filter prior to working with solutions that have extreme pH.
Hydrophilicity or Hydrophobicity
Filters used for aqueous solutions should be “wettable” or hydrophilic, otherwise you’ll be fighting an uphill molecular battle to get the solution to pass through the membrane.
Because membrane filters’ pores act like capillaries, water spontaneously enters and fills pores in hydrophilic membranes. After being wetted, the membrane will not allow air or gases to pass through.[5]
The most common membranes for biology applications are made of cellulose, nylon, and polyethersulfone (PES). A modified hydrophilic version of polyvinylidene fluoride (PVDF) is made to improve wettability for applications like Western blots.
Conversely, membranes can be made of hydrophobic materials. When water comes into contact with a hydrophobic membrane, the water is actively repelled from the pores and does not enter via capillary action like in hydrophilic membranes. However, by using a low surface tension fluid such as alcohol, it is sometimes possible to displace the air in pores and remove surface tension, thereby allowing water to pass through. This process is known as “pre-wetting”.
Common hydrophobic membranes used by biologists include those made of PVDF and polytetrafluoroethylene (PTFE), which are useful for working with organic solvents. Hydrophobic membranes are also commonly used for filtering gases and in venting applications because hydrophilic filters block airflow if they are accidentally wet by water splashes or condensation.
Throughput
Flow rate and filtration efficiency may not matter much for very small volumes, but large volumes require a bit more thought and planning to ensure you aren’t stuck in the lab until midnight. Moreover, particulates in samples can slowly build up, leading to “fouling” or clogging of the membrane.
Some membrane filters are asymmetric, meaning the top or feed-surface pores are wider than the pores at the bottom. This allows for faster flow rates and filtration capacity because the top part of the filter acts as a pre-filter to remove larger particles. It is particularly important to use these filters in the correct orientation, so they are not susceptible to early clogging.
As a general rule, increasing filter surface area leads to particulates being better distributed across the area during filtration, leading to increased throughput.
Extractables or Leachates
The last thing you want to find out after you use a filter—especially those you plan to use for a sensitive chemical analysis like HPLC or MS—is that your filter has contaminated your precious sample with material from the filter itself. Extractables can arise from fiber particulates being shed during filtration (especially in clarification filters) or from residual chemicals used to manufacture the filter. If you’re worried about extractables, you can compare your samples with a control by running just the solvent through the filter and analyzing the resulting filtrate.
We hope this gets you thinking critically about filter choice and use in the lab. Do you have additional advice or considerations for filtration? Let us know in the comments below!
Resources
1. Brose DJ, et al. (2002) Membrane filtration. Pharm Biotechnol.14:213–79.
2. Mountain Empire Community College. Water/Wastewater Distance Learning Website. (Accessed November 26, 2021)
3. Binding Properties of Filters. Merck Millipore. (Accessed November 26, 2021)
4. Millipore. Microfiltration Membranes for the Laboratory. (Accessed November 26, 2021.)
5. Sterlitech Corporation. Principles of Filtration. (Accessed November 26, 2021.)







