What do cell lysis, clean dishes, and gallbladders all have in common?
Answer: detergents!
These useful chemicals can solubilize fats and other proteins in water. They are the key to applications as varied as lysing cell membranes, extracting DNA, and solubilizing proteins for gel electrophoresis.
To help you understand these important chemicals, we provide a quick refresher on what detergents are, biochemically-speaking, along with an overview of different detergent types.
What are detergents?
Detergents have a tadpole-like structure, in which the “tail” is hydrophobic and the “head” is hydrophilic. In water, detergent molecules aggregate to create sphere-like “micelles,” with all of the heads facing out and the tails facing in. Micelles can form around proteins, with the protein’s hydrophobic residues interacting with the detergent’s hydrophobic tails. By encasing proteins in a hydrophilic micelle, detergents effectively solubilize hydrophobic chemicals in water.
To form a micelle, there have to be enough detergent molecules in solution – a critical micelle concentration. The number of molecules needed to form a micelle varies by the type of solution, salt concentrations, pH, and temperature. Generally speaking, detergents with longer tails need fewer molecules to reach the critical micelle concentration.
But while the tail is important, it’s a detergent’s hydrophilic head the plays the key biochemical role in molecular biology.
Types of detergents
1. Ionic
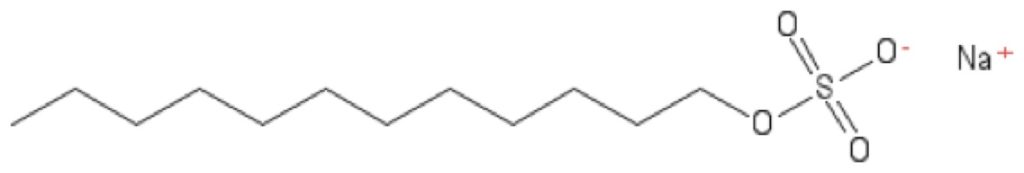
Ionic detergents have positively- or negatively-charged heads. This charged head makes them more “harsh” than neutrally-charged detergents, meaning they disrupt bonds between proteins. This effectively destroys the protein’s structure, which is why ionic detergents can deactivate enzymes and antibodies. But while ionic detergents are very good solubilizers, their charged head makes them more sensitive to pH and ions present in solution.
Sodium dodecyl sulfate (SDS), commonly used in cell lysis and gel electrophoresis, is an example of a harsh, ionic detergent with a negatively-charged head.
2. Non-ionic
Non-ionic detergents have uncharged, hydrophilic heads, making them milder than ionic detergents. They disrupt the bonds between lipids and proteins, but generally don’t disrupt protein-protein bonds. Enzymes and antibodies generally remain intact and functional in solutions of non-ionic detergents, which explains why Triton X-100 and Nonidet P-40 are so commonly used in lab. Unlike ionic detergents, however, non-ionics are more resistant to the effects of different ions (which also explains why hard water doesn’t affect non-ionic dishwashing detergents).
Nonidet P-40 is an example of a mild, non-ionic detergent with an uncharged, hydrophilic head.
Most non-ionic detergents fall into three different structural types. The bile salts – like those secreted by the gallbladder to emulsify fats – have a steroid structure, which has a polar orientation that acts as the head, and an apolar orientation that acts as a tail. The poly(oxyethylene) ethers, like Tween, have a neutral head with tails that are oxyethylene polymers. And the glycosidic detergents have hydrocarbon tails, but use carbohydrates like glucose for their polar heads.
3. Zwitterionic (also known as amphoteric, ampholytic)
Zwitterionic detergents, like CHAPS and Zwittergent 3-14, have a net neutral charge across the whole detergent molecule, making them similar to non-ionics. However, like ionic detergents, they disrupt protein-protein bonds – although zwitterionics are generally less harsh and denaturing than the ionics.
Zwittergent 3-14 is an example of a zwitterionic detergent: its net molecular charge is zero.
Using (and choosing) detergents
Detergents are invaluable tools in the lab (and the kitchen). But with so many detergents out there, how do you know which ones to use? In our next detergent article, we’ll cover some common uses of detergents in the lab, and offer some tips on choosing the right kind of detergent!
References:
- Coligan JE. Appendix 1B: Commonly Used Detergents. Current Protocols in Molecular Biology. 1998. John Wiley & Sons, Inc.
- Caligur V. Detergent Properties and Applications. 2008. Sigma-Aldrich.
- Preparing Samples for Western Blot Analysis: Cell Lysis. 2014. Advansta Wiki.
- Ophardt CE. Virtual Chembook: Detergents and Surfactants. 2003. Elmhurst College.
Images made in PubChem Sketcher V2.4.