Just like you need to clean up your room from time to time, your cells also need to do a bit of housekeeping. Your cells accomplish this through a process called autophagy. Autophagy mainly serves two roles. The first is to remove damaging materials, such as misfolded proteins, dysfunctional organelles, and foreign invaders. The second is to breakdown cellular materials for energy during starvation.
Why Is Autophagy Important?
Improper regulation of autophagy is a potential factor for many diseases, such as cancer, diabetes, liver disease, autoimmune diseases, and infections. Not only does it remove damaging materials—it also stimulates senescence and helps the cell present antigens on its surface. Researchers have begun to recognize autophagy as an important process in both physiology and pathology. In fact, the 2016 Noble Prize in Physiology or Medicine belongs to Yoshinori Ohsumi for his work on autophagy.
Mechanism of Autophagy
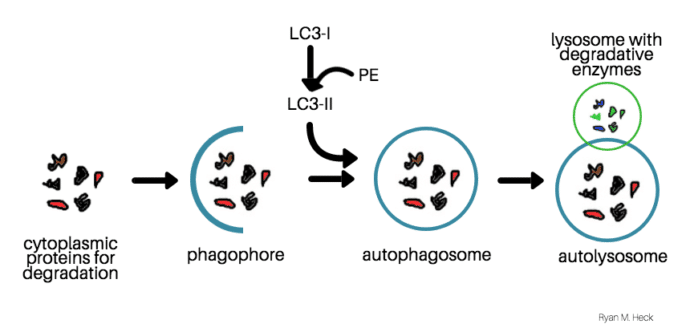
Autophagy begins when a membrane called the phagophore forms around materials that the cell needs to degrade. Simultaneously, the microtubule-associated protein (LC3-I) is conjugated to phosphatidylethanolamine (PE), and forms LC3-II. Then, LC3-II enters the membrane, which promotes the formation of the autophagosome. The autophagosome fuses with lysosomes to degrade the material inside. LC3-II is also degraded at the same time, making LC3-II a good marker for autophagy.
Quantify Autophagy by Measuring the Flux
If LC3-II is a marker of the number of autophagosomes present, can we just run a western blot and detect the amount of LC3-II present to quantify autophagy? No! Autophagy is a dynamic process—with autophagosomes constantly forming and disappearing. Therefore, the levels of LC3-II present potentially mean two different things.
Let me explain. If you see an increase in LC3-II on a western blot, that could mean the cell has increased autophagy because there are more autophagosomes. However, it could also mean that the cell has decreased autophagy because something is inhibiting the degradation of the autophagosome. The same holds true if you see a decrease in LC3-II. That could mean fewer autophagosomes are formed (less) but it could also mean that more autophagosomes are degraded by lysosomes (more).
Therefore, one of the best methods of quantifying autophagy is to measure the autophagic flux. This is how many autophagosomes form and then become degraded. To measure the flux, you need to detect the LC3-II turnover, or the difference in LC3-II levels in the presence versus the absence of lysosomal inhibitors.
Autophagic Flux = LC3-II levels (with inhibitors) – LC3-II levels (without inhibitors)
Different Types of Lysosomal Inhibitors
You can chose from many different types of lysosomal inhibitors to measure autophagic flux. Here are some of the most common currently in use:
- Bafilomycin A1: inhibits the fusion of autophagosomes and lysosomes
- Ammonium Chloride or Chloroquine: alters the pH of the lysosome, which inhibits the degradative activity of lysosomal enzymes
- E64d + pepstatin A: inhibits lysosomal enzymes
What Do We Use to Ultimately Measure LC3-II Levels?
If you are working with cell cultures, one option is to lyse your cells, run a western blot, and detect LC3-II with an antibody. You can also work with a cell line that stably expresses GFP-LC3 and count the puncta present on cells. If you are looking to do in vivo experiments, GFP-LC3 transgenic mice also exist.
Autophagy Is a Dynamic Process
It is important to view autophagy, not as a static, but a dynamic process. To quantify it, measure the flux by detecting the difference in LC3-II levels in the presence vs the absence of lysosomal inhibitors. For cell cultures, don’t forget your controls, because you will need two wells for each treatment, one with and one without inhibitors.
Best of luck with your quantification of autophagic flux and please comment if you know of other inhibitors or methods of measuring autophagy!