While there are many more methods to choose from for cleaning up your RNA or DNA than there used to be, sometimes Phenol-Chloroform extraction is still the best way to go.
Here I’ll discuss some of the practical aspects of using this technique.
A (very) Brief Guide to How Phenol-Chloroform Extraction works
Our previous article on how phenol extraction of DNA works touched on some of the ideas about how organic extraction will remove proteins from an aqueous solution.
In brief, proteins consist of both hydrophobic and hydrophilic residues, and through protein folding, achieve a compromise with water to remain soluble.
However, when they are given the opportunity to transition to an environment that can accommodate both polar and non-polar residues (i.e., phenol or phenol-chloroform) with no compromise required (i.e., folding), they happily move over to that phase.
The more highly polar molecules, like carbohydrates and nucleic acids, are “happier” in the aqueous phase (with some exceptions noted below) and remain there. Now for the nitty-gritty.
Phenol vs. Phenol-Chloroform vs. Chloroform
One of the most frequent questions I’m asked when training someone is what the differences are between the different organic phases used in phenol-chloroform extraction. Here is the breakdown, as best as I understand them.
Phenol
What we are actually talking about here is buffer-saturated phenol, which consists of a solution that is actually about 72% phenol, 28% water.
Since phenol is a weak acid, the solutions that we use have been equilibrated with buffer to bring the pH to a particular target—either acidic for RNA purification or slightly alkaline for DNA purification.
In addition to a certain amount of water dissolving into the phenol, there is a certain amount of phenol that dissolves into the water—at equilibration, the aqueous phase will contain about 7% phenol.
This is thought to aid in the extraction, as this dissolved phenol helps denature proteins while they are still in the aqueous solution. Buffer saturated phenol has a density that is only slightly higher than that of water.
Phenol-Chloroform
This is a mixture of buffer-saturated phenol and chloroform, usually close to 1:1 for DNA purification with other ratios sometimes used for RNA purification. Isoamyl alcohol is sometimes included as an anti-foaming agent but is generally thought to be an inert and optional addition.
This solution is commonly used in lieu of buffer-saturated phenol for a couple of reasons. As I mentioned above, the density of buffer saturated phenol is only a little higher than water. So if your aqueous phase contains enough salt or any other solutes that would increase its density, then you could end up with phase inversion during extraction, where your aqueous phase is under the phenol, rather than on top of it.
Chloroform is significantly denser than water, so adding it to the organic phase increases the overall density of that phase, helping to prevent phase inversion.
In addition, the chloroform (and some say isoamyl alcohol) helps reduce the interphase—the fuzzy border between the two phases populated by molecules that can’t decide where they want to go.
These can be partially denatured proteins, DNA (depending on the pH), and/or partially denatured DNA binding proteins that are still clinging to DNA, and it is a real pain in the butt.
If you pipette off some of this material when removing the aqueous phase, then you decrease the purity of your sample. If you are too timid while pipetting, then you hurt your yield.
If you have my luck, then whatever it was you wanted to keep most is sitting in it. Adding chloroform to the mix helps reduce this. (But I have an even better solution to this problem that I’ll tell you about below.)
Chloroform
This is normally used after phenol or phenol-chloroform extractions. While pure chloroform doesn’t work as well as the organic solutions mentioned above for protein extraction, it works well for extracting phenol from aqueous solutions.
Remember when I said that the aqueous phase contained ~7% phenol after equilibration? Do you also remember when I said phenol likes to denature proteins? If you don’t get rid of (or at least severely reduce) the phenol in your now protein-free nucleic acid solution, it could come back to haunt you by partially or completely inhibiting enzymes that you treat the DNA or RNA with down the line.
Presented with a nice chloroform home, however, the phenol will partition away from your nucleic acids. Chloroform itself is about 10X less soluble in water than phenol (~0.8%) and is less denaturing to proteins. I was also told long ago that it is less likely than phenol to pellet during ethanol precipitation of DNA once upon a time, but I cannot find a reference to back that point up.
Ether
This can also be used to extract phenol back out of the aqueous phase. However, because of the explosive potential of ether and the tendency of biology-types to have Bunsen burners and strikers in their labs, it has been largely replaced by chloroform.
Not so Pretty in Pink
A note of caution: don’t use your phenol or phenol-chloroform if the solution is turning pink.
Oxidation of the phenol produces pink/brown oxidation products, and these can cause nicking of your DNA and degradation of your RNA.
Most commercial phenol solutions contain an antioxidant to inhibit this oxidation, and phenol buffered at an acidic pH seems to be resistant to oxidation, but it isn’t a bad idea to move a portion of the buffer-saturated phenol (from the brown bottle that it likely came in) to a clear bottle or tube to inspect it before you start your extraction.
pH matters—a lot
Occasionally somebody does a phenol-chloroform extraction and doesn’t recover any of the DNA in the sample. If this happens to you, or somebody in your lab, your first question should be “Which phenol did you use?”.
Labs that do both DNA and RNA work will likely have both acidic and basic buffered phenol solutions, or somebody will buy a new bottle of phenol without paying attention to the pH.
Extraction of DNA containing samples with acidic phenol results in the denaturation of the DNA, and once denatured, the DNA partitions to the organic phase. This is a key feature of many RNA purification protocols, which is one of the reasons acidic buffer-saturated phenol is used.
For a more detailed explanation of why, read our article on Acid Phenol Chloroform Extraction of DNA, RNA and protein: 3 in 1.
Determining the pH of Phenol
Now, sometimes a lab’s DNA phenol extractions start failing (no recovery of the DNA afterward) and the pH of the phenol is called into question. If you find yourself in this spot, you can’t simply dip your pH meter into it, and you cannot use pH paper, since the pH indicator on the paper was characterized in aqueous solutions.
The method that I’ve used is to dilute 1 ml of the buffer saturated phenol with 9 ml of 45% methanol, mix, and then measure the pH with a standard pH meter.
Adjusting the pH of Phenol
The safest way of adjusting the pH is by replacing the aqueous phase on top of the phenol solution with a fresh aliquot of ~100 mM buffered water (usually Tris pH 7.9 for DNA work), mix the phases well, and then let the bottle settle until the phases are well separated again. Then pH it again.
Mixing your phases
Phenol-chloroform extractions are amazingly efficient—less than 1% of the average protein remains in the aqueous phase after the first extraction has come to equilibrium. The trick is to get the extraction to equilibrium, of course.
The more surface area there is between the two phases, the faster this happens, and that surface area is greater the finer emulsion you have created. This can be achieved by vortexing the phases for a couple of minutes, as many protocols call for, but not all samples can be vortexed.
If you are purifying very large DNA, like genomic DNA, then you may have to mix your sample much more gently, and therefore perform each extraction for much longer. So on this point, follow your protocol and be very cautious about trying to shave time off this step.
Effects of Denaturation and Digestion
Some protocols call for protein denaturation and possibly digestion with Proteinase K before phenol-chloroform extraction. Both of these steps are attempts to reduce the amount of material that is trapped in the interphase and therefore improve the yield of DNA or RNA recovered.
I have never seen any negative effect of denaturing the proteins with SDS before extraction. On the other hand, digestion of the protein could reduce the purity of the nucleic acid that you recover.
While whole proteins are almost guaranteed to partition to the organic phase, once the protein is digested into small peptides, not all of those peptides will have the same chemical ‘character’ of the whole protein—and each will have its own partition number.
It may not matter a lot if you have some peptides in your nucleic acid, depending on your downstream application, but it’s formally possible that these contaminants could affect your future quantitation of the sample. However, I discovered a better way of eliminating the dreaded interphase.
Phase Lock Gel™
This is one of those things that seems to be in 50% of labs, yet less than 10% of the people I’ve talked to know what it is or how it works.
I discovered this at a critical point in my research, and it saved my thesis.
In short, Phase Lock Gel is a gooey, Vasoline-like gel that has a density that is slightly greater than water. If you add your extraction on top of it in a centrifuge tube and then centrifuge it, the Phase Lock gel collects between the aqueous layer and organic phase, separating the two and preventing the formation of the DNA/RNA hungry interphase during phenol-chloroform extraction.
A search of the internet didn’t turn up any pictures of this process that I thought were good enough, so I took some of my own (Figure 1). In this little demonstration, red dye is taking the place of our precious nucleic acid, and blue dye is substituting for protein.
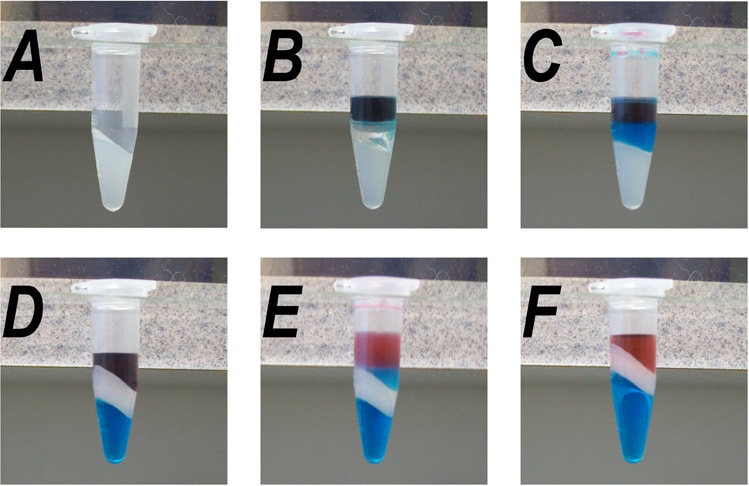
Figure 1: Use of Phase Lock Gel™ during phenol-chloroform extraction. (A) The Phase Lock gel® was pelleted into the bottom of a 1.5 ml Eppendorf tube. (B) After adding phenol-chloroform and the aqueous phase, complete with faux DNA (red) and faux protein (blue) in the aqueous phase. (C) After gentle shaking for 5 minutes. (D) After centrifugation. Note the gel now separates the organic phase from the aqueous phase. (E) After a second addition of phenol-chloroform and gentle shaking for 5 minutes. (F) After the second centrifugation. The faux DNA could now be extracted with chloroform (in the same tube, if space allows) to remove the residual phenol.
As you can see, the gel forms a stable partition between the two phases, and if you want to extract the sample a second time and there‘s still room in the tube, then you can do it, using the same tube two or more times with no compromise of the sample purity.
You cannot vortex mix the two phases in a tube containing this reagent, but you can vortex mix in a separate tube, then add the sample to the tube with the gel and centrifuge. They have this gel in two different flavors (please don’t eat!)—one for regular samples (light) and another for high-density samples, like solutions with high salt or protein concentrations (heavy).
Using SDS to denature the proteins in my sample prior to extraction and then employing Phase Lock Gel™ to separate the phases has consistently given me DNA samples with 260/280 ratios of 1.8 and greater than 98% recovery. Seriously great stuff.
Vacuum Grease as an alternative to Phase Lock Gel™?
Thanks to Roberto Rosati for uncovering a paper showing that silicone lubricant (aka vacuum grease) has been successfully used to help aid the recovery of nucleic acids.
The addition of this grease (which is non-toxic and autoclavable) resulted in a tight interphase allowing total recovery of the aqueous phase. (1)
We even had an enthusiastic Bitesize Bio reader test out the method during their phenol-chloroform extractions—read his report on how successful vacuum grease is as a DIY phase separating gel.
In preparing this article I came across this website, which has a lot of useful information. Visit it if you would like to learn more about phenol.
A Note of Caution
It’s easy to become complacent about the chemicals we use in the lab, especially when we use them regularly. Phenol is a particularly unpleasant chemical and is both toxic and corrosive.
Make sure you use appropriate PPE, including suitable lab gloves and safety glasses, and know what to do in case of a spill or accident.
Now to hear from you: what are your tricks and tips for perfect phenol-chloroform extractions?
Originally published 3 May 2010. Reviewed and updated October 2021.
References
1. Mukhopadhyay T, Roth JA. (1993) Silicone lubricant enhances recovery of nucleic acids after phenol-chloroform extraction. Nucleic Acids Res. 21(3):781-782.







