If you ever worked in a biology or biochemistry laboratory, you probably already heard about ELISA. You may have even used it. But do you know what’s behind it? And how you can improve it? Let me guide you through the basics of ELISA, and introduce you to my favorite ELISA technique—AlphaLISA.
First Things First…
So, what is an ELISA? ELISA stands for “Enzyme-Linked Immunosorbant Assay.” You can probably guess from the string of jargons above that the assay might involve some kind of immune-globulin linked to an enzyme for capturing of certain antigens? Bingo! That’s exactly what it is. ELISA is an assay developed to detect the presence of your “protein of interest”, or the antigen, using monoclonal antibodies. Read this article on the ELISA technique to learn more.
ELISA has been extensively used in many clinical applications, such as medical diagnosis.1 For example, an in-home pregnancy test essentially is an ELISA that detects the presence of human chorionic gonadotrophin (hCG) in the urine. In labs, ELISA is an indispensible technique when it comes to protein detection and quantitation. Compared to western blot analysis, the ELISA is high throughput and quantitative.
However, such an assay has several drawbacks… Imagine the time it takes to add liquid into a 96-well plate using a multi-channel pipet and repeating this 3 or 4 times in each step! In addition, you’re constricted to a particular “time window” to obtain your result, because even if you add a stop solution to halt the enzymatic reaction, the background noise will increase over time. Don’t worry, there is always a new way! In the next section, I will introduce you to a different kind of ELISA called AlphaLISA, which is based on a very different chemistry and physics. This technology will not only cut your traditional ELISA assay runtime in half, but it also works on other applications (such as detection of protein-protein interactions, screening of antibody pairs, and pin-pointing key components of the signal transduction pathways).
Discover AlphaLISA
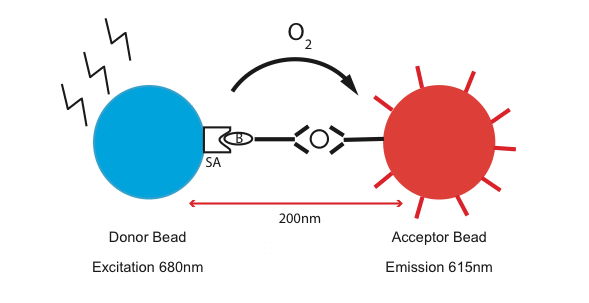
So, what’s so cool about AlphaLISA? It stands for “Amplified Luminescent Proximity Homogenous Assay.” Simply, it uses two different kinds of beads, one called the “donor” and the other one called the “acceptor”, to detect the closeness of an interaction. The streptavidin-coated “donor” beads bind biotinylated “capturing” antibodies that recognize a specific protein (Fig 1). The acceptor beads can be conjugated with secondary antibodies that also recognize the same protein, but in a different region. As a laser beam is shone on the donor beads, a singlet oxygen molecule is generated. This leads to an excitation and amplification of the fluorescent signal on the “acceptor” beads. Sounds simple right?
One of the biggest differences with the traditional assay is that AlphaLISA is a proximity assay. As you can see in Figure 1, the donor and the acceptor beads have to be within 200 nm of each other for the signal to get across and amplify. This makes AlphaLISA a great tool for studying protein-protein interaction.2 Another great thing about this assay is that it requires half the amount of time than the traditional ELISA because of a lower number of washing steps! AlphaLISA, a very versatile assay, can cover a wide range of protein sizes, from large endogenous proteins complexes to very small peptides.3 In addition, it works in a variety of sample types such as serum, plasma, cell lysates & supernatant.3
AlphaLISA in Five Simple Steps!
Step 1. Add analyte to a 96-well microplate.
Step 2. Add biotinylated antibody that recognizes the protein. Also, add the antibody-conjutaged acceptor beads into the wells. Incubate for 60 min.
Step 3. Add streptavidin-coated donor beads for 60 min.
Step 4. Excite the donor beads with laser. The intensity of light emission is proportional to the level of interaction.
Step 5. The samples can be analyzed using automated plate readers equipped proper lasers to excite the beads and proper light detectors.
Clinical Applications of AlphaLISA
As you can see, the chemistry behind AlphaLISA makes it more versatile than ELISA. Here are some of the applications:
- Epigenetic modifications: You can monitor enzymes that control the status of the chromosome, which often shows different activation patterns in cancer cells or other pathologies.
- High throughput screening of biomarkers : This process is much less labor intensive and more efficient.
- Protein-protein interactions: If you want to find out the binding partner(s) of your “favorite” protein, then this assay would allow you to screen many candidates and quantitate that interaction (as opposed to immunoprecipitation).
- Cell signaling cascades: You can use a similar strategy as above if you are interested in the signaling cascades of a particular pathway
And Now?
I hope that are now convinced about how AlphaLISA is easy to use and highly versatile! Give it a try for your next experiment, and let me know in the comments how successful you were!
References
- Voller, A, A Bartlett, and D E Bidwell. “Enzyme Immunoassays with Special Reference to ELISA Techniques.” Journal of Clinical Pathology 31, no. 6 (June 1978): 507–20.
- Eglen, Richard M, Terry Reisine, Philippe Roby, Nathalie Rouleau, Chantal Illy, Roger Bossé, and Martina Bielefeld. “The Use of AlphaScreen Technology in HTS: Current Status.” Current Chemical Genomics 1 (February 25, 2008): 2–10. doi:10.2174/1875397300801010002.
- Beaudet, Lucille, Roberto Rodriguez-Suarez, Marie-Hélène Venne, Mireille Caron, Julie Bédard, Véronique Brechler, Stéphane Parent, and Martina Bielefeld-Sévigny. “AlphaLISA Immunoassays: The No-Wash Alternative to ELISAs for Research and Drug Discovery.” Nature Methods 5, no. 12 (2008): -. doi:10.1038/nmeth.f.230.