So, you’ve extracted your precious RNA and want to check its quality on a gel. Conventionally, you would run a formaldehyde gel, which is messy and requires a lot of prep. Plus, it is a huge undertaking in terms of time (and money) if all you want to do is just check the quality of your RNA.
Sure, you could also run a 1% agarose gel for that. But, you expose your RNA to omnipresent RNAses, which often results in smeared bands and leaves you confused if the degradation occurred during isolation or in the gel.
An alternative to formaldehyde gels
For those who don’t have the time to prepare formaldehyde gels, yet want a quick and reliable method, Aranda et al. have come up with a solution. In their article in the journal Electrophoresis, they present a protocol for checking RNA quality by incorporating bleach into agarose gels1.
Yes, you heard it right. Bleach – that funky smelling liquid, which gets rid of bacteria and germs and the like, that you use to clean sinks and bathrooms. It turns out, the active component of bleach, sodium hypochlorite (approx. 6%), can denature proteins through oxidation.
Now, this property has been put to good use against RNAses by incorporating a small amount of bleach into the agarose gel before melting and casting.
How to use bleach to defeat RNAse contamination
The procedure is deceptively simple: all you do is add 0.5% v/v of bleach into your agarose and 1% TAE mixture before you stick it in the microwave for melting. The rest of the procedure is similar to a conventional nucleic acid gel; you add your stain of choice (I use EtBr), cast the gel, and run it in 0.5X TAE at 50V or 100V.
The authors also tested bleach with TBE gels and ran DNA gels with bleach—both with good results. Bleach does not degrade nucleic acids.
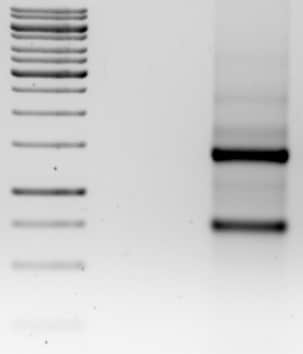
I used this method to check RNA quality before cDNA synthesis and the results are shown below; I saw crisp bands for the 28S and 18S rRNA with a 2:1 intensity ratio (third lane). Also, the 1kb DNA ladder (first lane) did not show any DNA degradation.
Points to note while using bleach
Bleach, in general, is more tolerable than formaldehyde. The volume of bleach used in the bleach gel is extremely low. Since the normal 6-lane gel requires around 30 mL of agarose, only 150 ?L of bleach needs to be added to the gel. The smell is almost untraceable upon melting the gel. However, sodium hypocholorite is known to react with ammonia to produce chloramines, which are toxic. Therefore, if you plan to run bleach gels on a regular basis, I would recommend wearing a mask and preparing the gel in a fume hood.
To check whether the bleach gel would be suitable for northern blotting, I tested the reactivity of a nitrocellulose membrane with bleach. I dropped a piece of the membrane into bleach and found that it raised quite a stink, which could indicate some sort of reactivity with the membrane. Although the volume of bleach used in the gel itself is quite less, I am not certain how it would affect a northern blot as the reaction between bleach and nitrocellulose is unknown.
If you have the habit of making agarose gel in bulk and repeatedly heating and cooling it, make the gel without bleach and add the bleach into the gel right before melting. However, I would recommend making the gel to requirement each time because making the bleach gel takes considerably less time. Also, repeated melting will result in an increase in agarose concentration, so the volume of bleach added to the gel will also need to be altered accordingly.
So, try out this cheap alternative and save time and money!
References
Aranda PS, LaJoie DM, Jorcyk CL. 2012. Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis. 33:366-9. https://dx.doi.org/10.1002/elps.201100335