Today, PCR is as common a feature to the lab as pipettes and beakers. The majority of us regularly need to amplify our DNA or RNA samples, sometimes for an ‘everyday’ PCR run just to check if our primers actually work, or in a quantitative (q)PCR run, where we might be comparing the levels of different mRNAs in response to a biological treatment or detecting SNP variants.
Despite being a PCR regular myself, one thing I know from experience is that when I’m juggling two or three experiments at a time and setting up primer optimization assays, my ability to perform basic mathematics is somewhat diminished. Optimizing multiplex assays for qPCR can also be a thankless task, and a lot of time-consuming trial and error is often required to get the assay up and running.
So I was really thankful to come across a fantastic new qPCR toolbox put together by the qPCR design lab from Biosearch. This site incorporates a number of convenient tools for helping researchers through their everyday PCR ‘oligo’ calculations, but what’s even handier is their guidance with multiplex qPCR experimental design (see section on Spectral Overlay).
These Toolbox features can do much of the pre-experimental work for you and are found all in a single place!
What is in the Oligo Toolbox:
OligoSpec™ Calculator
The OligoSpec™ Calculator determines the specific physical properties of your primer or probe, including any 5’, 3’ or internal modifications that you may have, such as fluorophores, Black Hole Quencher® (BHQ®) dyes, biotin labels, or even modified bases. This could come in handy if you know the sequence, but not the molecular weight (MW), and want to convert your stocks from ‘grams’ into ‘moles’. Also, this tool provides the extinction coefficient of the oligo at 260 nm, which can be used to quantify the stock concentrations in conjunction with the Concentration Calculator.
qPCR Reaction Estimator
The qPCR reaction estimator is a tool for calculating how long your little vial of primer or probe will actually last you…theoretically anyway, providing the estimated number of reactions you can expect to run with your stock. Of course, some extra volume will be lost here and there through pipetting, so make sure to take that into account.
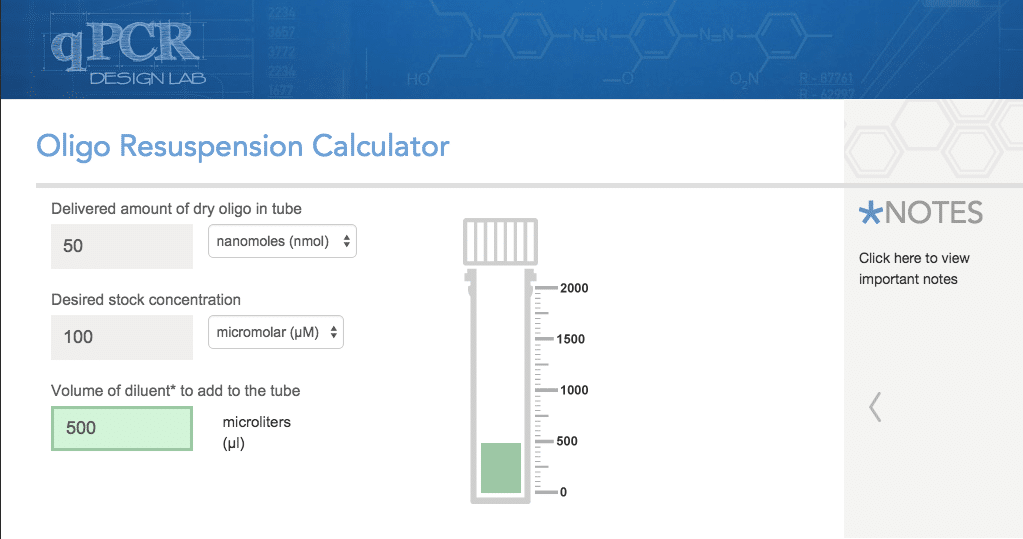
Oligo Resuspension Calculator
This is a helpful little tool to calculate the exact volume of water or TE buffer to re-suspend your newly delivered oligos. Some oligo manufacturers will only provide the volume of diluent to add to the dry primers to make a stock concentration of 100 ?M. However, this can be limiting and if you need to prepare a different stock concentration, then this tool is for you. While you’re at it, you should also consider aliquotting your oligos into a series of aliquots at working concentrations for a single day’s use, since multiple freeze–thaw cycles promote oligo degradation.
Oligo Dilution Calculator
Similar to the above tool, this is a foolproof calculator for those multi-tasking days when our math just isn’t up to scratch! Input the oligo’s stock concentration, the desired final concentration and volume, and ta-da, the volume of stock and required diluent is provided. One useful feature of the dilution calculator is the ability to convert to different units; no more worrying about counting decimal places switching from picomolar to micromolar.
Concentration Calculator
Quantifying your stocks may be important for the following reasons; 1) you mislaid the product insert detailing the amount (nmoles) of dry oligo in the tube (fortunately it’s usually printed on the side of the tube), 2) you (or another lab member) did not write the re-suspended primer concentration on the side of the vial, or 3) you want to double-check primer concentrations if there is concern over the quality of your primers, and you suspect that the full amount of dry primer has not been recovered or the exact primer concentration is really critical to your experiment.
In any of these cases, the Concentration Calculator tool could come in handy. By inputting the Optical Density at 260 nm (measured in the lab with a spectrophotometer) and the Oligo Extinction Coefficient, calculated using the OligoSpec™ Calculator mentioned above, the stock concentration can be deciphered.
Spectral Overlay Tool for Multiplexed qPCR
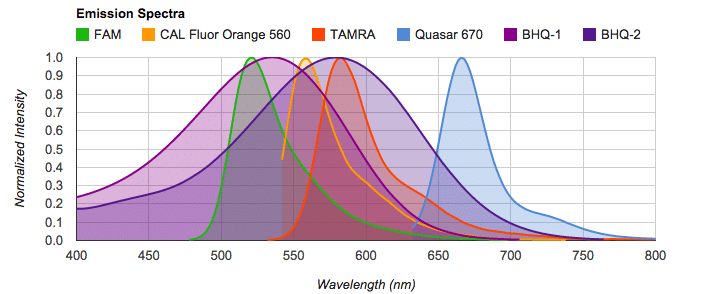
The spectral overlay tool is a neat, and, as far as I know, a unique feature of the Biosearch qPCR toolbox. It is particularly useful for designing multiplex qPCR experiments, which require multiple probes labeled with specific fluorophores (dyes) for detection of multiple qPCR products. Fundamentally, the different fluorophores must not emit excitation at the same wavelength so that they remain distinguishable from each other.
This spectral overlay tool displays the absorption and emission spectra for each available fluorophore and quencher, allowing the user to identify any overlaps. The tool also accounts for the compatibility of the dyes with the qPCR system being used (covering most manufacturers), so you can find the optimal set of fluorophores and quenchers for your instrument.
While that should be plenty to whet your appetite, spend some time checking out some added-value features that qPCRdesign.com has to offer. On the site, you can find qPCR design software and an array of constantly updated resources, including a reference library and a selection of videos and links all related to qPCR. Plenty to keep you busy!