Chemical chaperones are necessary in protein experiments. From buffers to storage solutions, chemical chaperones silently make proteins happy and soluble. Read this article to appreciate the love that chemical chaperones bathe on your proteins and learn when to use them!
A chemical chaperone is a molecule that promotes the favorable interaction of protein with water in a nonspecific manner.
As we all remember from Protein Chemistry 101 a major driving force in protein folding and solubility is the hydrophobic effect. If a protein isn’t folded correctly, hydrophobic regions of the protein are exposed causing aggregation, degradation and insolubility. Once your proteins-of-interest are extracted away from the natural folding systems of the cell (which centers on protein chaperones), they become susceptible to unfolding. To overcome this problem chemical chaperones are often added to extraction and storage buffers.
Chemical chaperones are naturally occurring molecules that are often upregulated in vivo during times of stress. They are amphipathic in nature: that is they contain both hydrophilic and hydrophobic properties. This allows them bind both to the hydrophobic sites on your protein and simultaneously hydrogen bond with the surrounding water of your buffer. This promotes protein solubility, and prevents aggregation and heat-induced denaturation.
Commonly used chemical chaperones include:
- Glycerol.
- Trehalose.
- Mannitol.
- Maltose.
- Sucrose.
- Glycine betaine.
- Phenylbutyric acid.
- Trimethylamine oxide.
- DMSO (dimethyl sulfoxide).
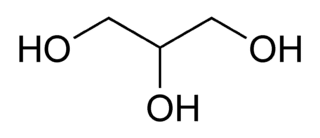
The most often used chemical chaperone is glycerol. Glycerol is often added to commercially purchased enzymes and proteins that are stored below freezing. Glycerol prevents freeze/thaw cycles by lowering the freezing point of the solution. However, glycerol is not just added to prevent freezing – lots of things could do that (such as ethanol) – glycerol is added because it also promotes protein stability by directly permitting energetically favorable interactions of the protein with water.
Another favorite atypical “chemical” chaperone choice is the protein BSA (bovine serum albumin). BSA is often added to protein preparations and enzymatic reactions, such as antibodies and restriction enzyme digests. I used to think excess BSA was added to samples just to prevent proteins-of-interest from sticking to the sides of the tube. But apparently BSA may be doing more than that! BSA has a propensity to non-specifically promote protein folding and reduce protein aggregation.
When You Should Use Chemical Chaperones
You should consider giving a chemical chaperone a whirl if things just aren’t working out with your…
- Protein purifications.
- Protein/protein interaction studies.
- In vitro enzymatic reactions (restriction enzyme digests, phosphorylations, etc).
- Antibody experiments.
- Protein crystallization experiments.
And of course you may need to use a chemical chaperone as a control if you are studying protein folding and/or chaperone functions.
When You Should NOT Use Chemical Chaperones
So, as with anything, there is a downside to using chemical chaperone. Some argue that the use of chemical chaperones alters native protein structure, thereby compromising the biologic relevancy of your experiment. So bear this in mind if you do decide to use a chemical chaperone.
Other (non-chemical) chaperones
In addition to chemical chaperones, there are several other methods and manipulations to promote protein folding. These include:
- pharmacological chaperones, which are designed to promote the folding of a specific protein or to lock them into a stable form
- upregulation of endogenous folding systems using chemicals or heat, which in turn promote global protein folding.
What kind of chemical chaperone you choose is up to you. The best chaperones are usually found experimentally, so I encourage you to experiment with them. A chemical chaperone just might be the difference between a failed experiment and a successful one.
Good luck, and keep your proteins happy anyway you can!