Cell confluency can affect cell behavior and growth, so it’s important to accurately and reliably measure cell confluency. But what is confluency? How do you measure it? And why is it a crucial consideration for your experiments?
What Is Cell Confluency?
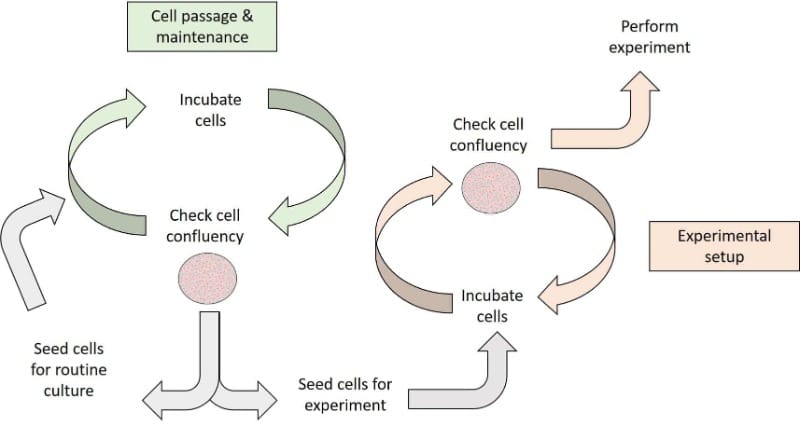
An efficient cell culture workflow is vital in many research areas in the life sciences and the biopharmaceutical industry and involves checking cell confluency.
Importantly, cell confluence isn’t the same as cell number. Instead, it’s defined as the percentage area covered by adherent cells in a culture dish or flask. (1) This is a routine measurement used to track cell proliferation during cell culture.
Cell confluence is important for determining timings for splitting (or passaging) and harvesting cells and for drug treatments or differentiation experiments (Figure 1). Accurate and reproducible measurements are vital for generating high-quality, reliable data. In industry, accurate measurements are especially important for standardizing cell culture protocols for developing and manufacturing cell therapies. (1,2)
Why Is Cell Confluency Important?
High levels of confluency can dramatically affect cell behavior and culture kinetics. (1) For example, at high confluency, myoblasts and preadipocytes can spontaneously differentiate into myotubes and lipid droplet-containing adipocytes, respectively.
Differentiation can also affect the proliferative potential of cultures. This means that if cells need to be cryopreserved for later use, they should be frozen at subcritical confluence. This would be 70% for myoblasts, but this value is dependent on the cell line. (1)
Effect of Confluency on Cell Health
Cell death can happen at high confluency because nutrients in the media become depleted or cells start competing for space on the culture dish or flask surface. Therefore, if cells are harvested and cryopreserved at a high or critical confluence, many or even all of the cells may die once thawed.
If this happens, you might have to use more of your cell stocks, and your cells may take longer to grow and reach the required confluence. Either way, this can cause huge headaches and be a massive waste of your time and resources.
Drug Discovery and Confluency
It’s also important to consider cell confluency in cell-based drug discovery projects. One of the most important sets of experiments in my PhD relied on using cells at low confluency and treating them with a drug to determine efficacy. It was imperative to use cells at low confluency to exclude any non-specific effects caused by over confluent cells and not from the test drug.
Effect on Morphology
Cell confluency is also a vital consideration if you’re studying cell morphology over time. Some cells may have one morphology at low confluence and a different one at high confluence. For example, NIH3T cells are flat and elongated at low confluence, but when they become confluent (i.e., 70 – 80% confluence), they re-arrange into an organized brick-like monolayer, which affects other cell characteristics. (1)
How to Determine if Your Cells Are Confluent
Given its importance in cell-based experiments, there are various methods for measuring cell confluency. However, currently available techniques are often subjective, complex, destructive, and/or time consuming. Some of these methods are summarized in Table 1.
Method | Advantages | Disadvantages |
Chemical dyes | Relatively fast and can be measured on a plate reader (colorimetric or fluorescent) | Indirect measure of confluency |
Qualitative visual measurement | Label-free | Subjective and can vary depending on the researcher(s) doing the visual inspection |
Image processing methods (e.g., Olympus CKX53 culture microscope, CKX-CCSW confluency checker software, and the Air Fraction (AF) output (3) | Accurate, objective, and consistent | Can require expensive and specialized microscopy equipment and software algorithms |
Table 1. Methods for measuring cell confluency.
What Is Meant By the Percentages?
Cell confluency is typically given as a percentage, which refers to the proportion of the culture dish or flask covered by adherent cells (e.g., HEK293 cells). For example, if cells are around 80% confluent, a monolayer of these cells covers 80% of the surface of the culture dish or flask. If you are not experienced in measuring confluency, it can be a difficult guessing game, especially if you are using qualitative visual measurements. Below are some simple rules and graphical representations to help you with this.
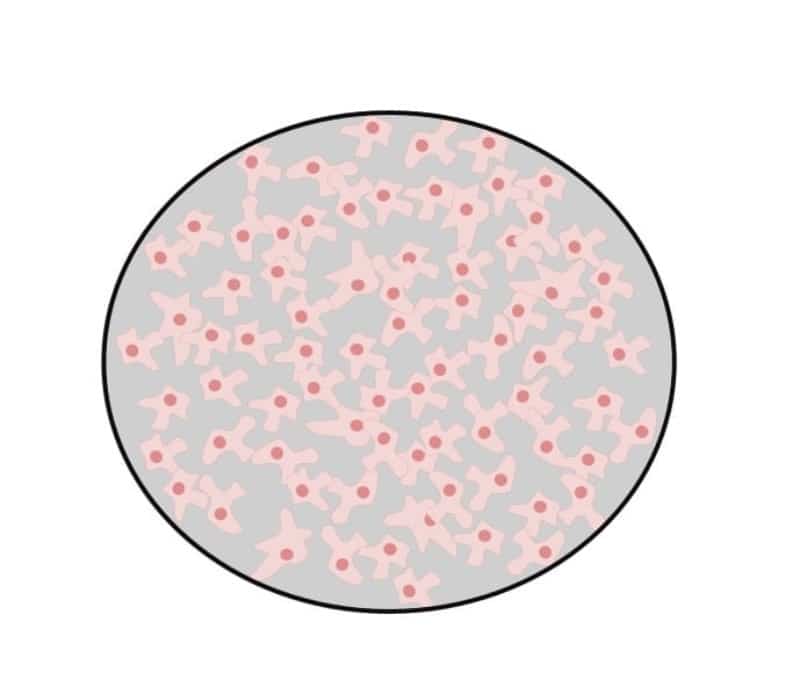
50% confluent
This is a relatively easy estimation because the area covered by cells should be similar to the area not covered by cells (Figure 2).
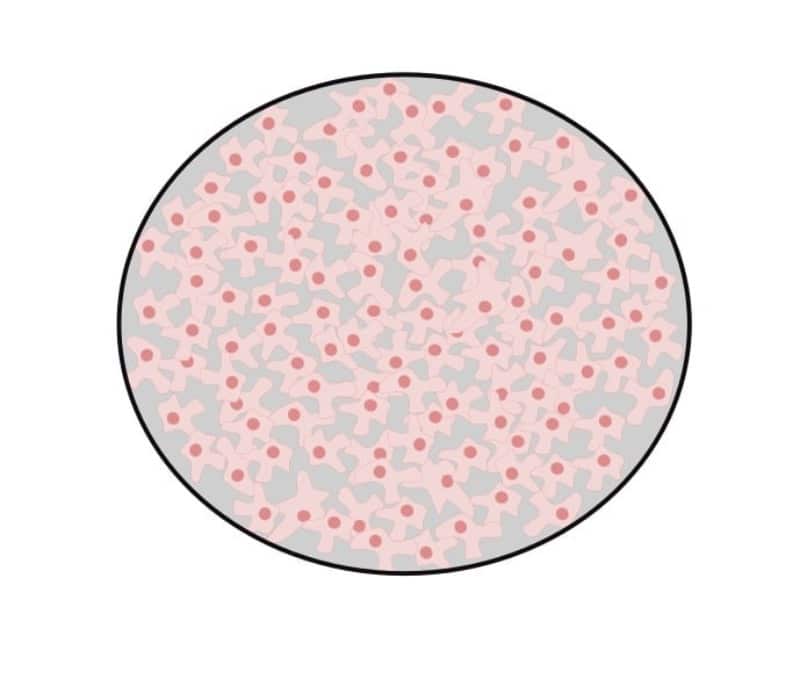
70% – 80% confluence
At this confluency, cells should still be growing exponentially but nearing the end of log-phase growth. They will cover most of the dish, but gaps are still present (Figure 3).
Ideally, cells should be split at this stage because this will improve overall cell viability. Splitting your cells at this stage will also lead to less aggregated cells. These cells will also have a shorter lag time (i.e., the time taken before cells start logarithmic growth) after they are split and/or thawed after cryopreservation.
100% confluence
Again, this is another simple estimation because, in this case, the entire surface of the culture vessel will be covered by cells with no space visible between them (Figure 4). If cells reach this level of confluence, one of two things will happen:
- Normal cells have contact inhibition and will rarely reach 100% confluence.
- Immortalized cells don’t have contact inhibition, which means that when they’re 100% confluent, they’ll squeeze together, and crowding will cause cells to reduce to half of their original size. If these cells aren’t split, they’ll start detaching from the flask surface, leading to cell death.
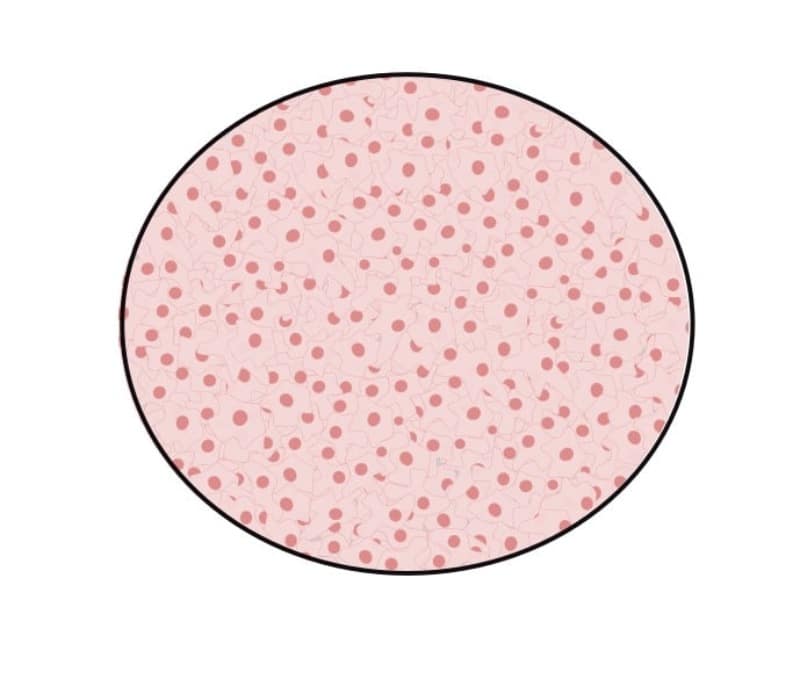
Generally, you should avoid letting your cells become over confluent because they’ll likely start to die off (sometimes very quickly). They may not be recoverable, especially after being stored in liquid nitrogen for a long time. Read our “What’s in a Number: Getting the Right Passage in Cell Culture” article to learn more about how and when to split your cells.
Tools for Estimating Confluency More Accurately
Once you become more experienced at estimating cell confluency by qualitative visual inspection, it’ll become easier, and your measurements will (hopefully) become increasingly accurate. Nonetheless, automated image-based methods are the gold standard for accuracy, reliability, and reproducibility. Examples of such tools are given below.
Air Fraction (AF) Output
This method uses a simple cardboard coverslip that can be cut to size to fit different culture flasks. Cells are imaged using an inverted phase-contrast light microscope, and images are captured with a digital camera equipped with a camera lens adaptor. Images can then be analyzed for confluence using the free ImageJ software. The output is a measure of confluence, referred to as an Area Fraction (AF). (3)
The Personal AUtomated Lab Assistant (PAULA)
The PAULA from Leica Microsystems can be used on the bench, in the hood, or even in the incubator, allowing you to study your cells in their natural state. In this way, the cells undergo less stress which is always good! The PAULA takes images in seconds, allowing you to check the confluency of multiple flasks of cells in seconds rather than spending hours at a regular microscope.
If you’re studying large cell populations with different fluorescent markers, or if you’re studying morphological changes over time, the PAULA will be useful for you as it supports multi-fluorescence and time-lapse imaging of cells under physiological conditions in the incubator.
For added convenience, the PAULA also connects wirelessly to any Windows, iOS, or Android compatible phone or tablet, allowing you to check the status of your time-lapse in between coffees and experiments.
Olympus CKX53 Culture Microscope and CKX-CCSW Confluency Checker
Cell confluency can also be accurately measured using a combination of the CKX53 culture microscope and CKX-CCSW confluency checker software. Unlike with the PAULA, cultures need to be taken out of the incubator for imaging, but this method is also non-destructive, meaning there’s no need to take samples from your culture flasks.
This helps to preserve cells for your downstream work, and this also limits the risk of contamination, all very important if you have a limited supply of cells for your experiments.
Here, the CKX53 microscope can be linked to the CKX-CCSW confluency checker software so you can visually inspect your cells and then capture images in one smooth workflow. The software can then accurately and reproducibly calculate cell confluency.
Cell Confluency Summarized
I hope I’ve given you an insightful overview of the importance of accurately measuring cell confluency. I’ve given you some examples of tools you can use to achieve this in the lab, but this is by no means an exhaustive list. If you have any tips and tricks you’d like to share, please do so in the comments below.
References
- Topman G, Sharabani-Yosef O, Gefen A. (2011) A method for quick, low-cost automated confluency measurements. Microsc Microanal. 17(6):915–22.
- Cell Confluency Tool. [Accessed 2022 Mar 20].
- Non-invasive and non-destructive measurements of confluence in cultured adherent cell lines Elsevier Enhanced Reader. [Accessed 2022 Mar 20].