If your research involves purifying a membrane protein, I sympathize with you. It’s an onerous task. After countless exhausting months in the lab, exiled from progress, you’ve returned to your supervisor clutching a reasonably pure sample of your target.
But it’s inactive, won’t crystallize, and is slowly precipitating out of solution.
It’s likely your problems are due to an excess of detergent. A small portion of detergent molecules keeps your membrane protein sample water-soluble, while the excess detergent blocks your assay substrate, convolutes your electron micrographs, and gums up crystallization interactions.
Let’s go through a few methods by which you can remove excess detergent from membrane proteins, and better yet, learn how to tell if you’ve been successful at it.
Why Is There Excess Detergent Anyway?
Unless you intend to use your purified membrane protein for a niche application, you’ve probably extracted it from the cell membrane using a detergent.
And you’ve learned somewhere along the way that you need to have the detergent present at 100x its Critical Micelle Concentration (CMC, the concentration at which detergent molecules self-associate into micelles) to extract your target from the cell membrane efficiently.
Because you’re smart, you’ve realized that a detergent concentration of 100x CMC is a lot, so you have incrementally reduced its concentration at every step of your purification procedure.
The problem is that most detergents form micelles that have a relative mass similar enough to that of your target protein to render separation of one from the other impossible. The consequence of this is when you spin-concentrate your membrane protein sample before your intended experiment, you usually co-concentrate the detergent micelles.
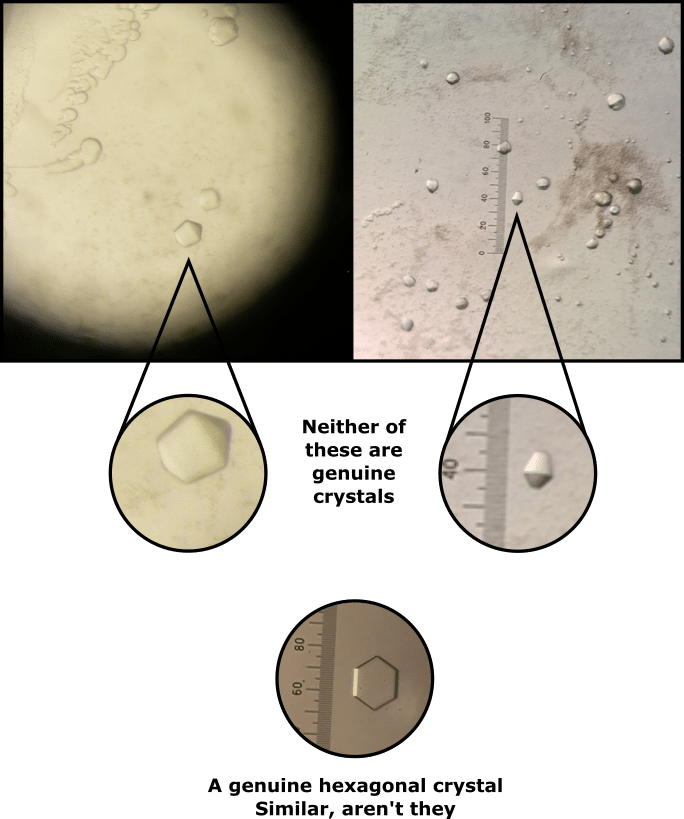
Fortunately, some techniques can help remove excess detergent from membrane proteins. Look no further than Figure 1 for an uncensored example of the heartache that excess detergent can cause a protein crystallographer. Don’t let the same happen to you.
6 Ways to Remove Excess Detergent From Membrane Proteins
1. Styrene Bead Adsorption
Porous polystyrene beads vacuum up detergent molecules through adsorption and are sold under the brand name “Bio-Beads.” Throw a couple of them into your sample and sequester that pesky excess detergent. [1]
Pros
- Very cheap and simple to perform, requiring only the beads and your sample.
- Bio-Beads are available through common chemical vendors.
Cons
- The optimal bead-to-sample ratio requires experimental determination, which in turn requires labor.
- Over-removal of detergent could precipitate your membrane protein sample out of solution by stripping away the detergent molecules that are actually solubilizing it. Over- and under-removal of detergent are both possible if the bead-to-sample ratio is not accurately determined.
- The beads will also adsorb lipids that may be essential to your sample’s function.
2. Size-Exclusion Chromatography
Anybody who has purified a protein has probably performed Size-Exclusion Chromatography (SEC). This technique separates molecules based on their hydrodynamic radius, which is usually a good proxy of their relative mass. You can therefore use SEC to separate excess detergent from your membrane protein sample. [2]
Pros
- The kit required to perform this technique is available in most protein research laboratories.
- Analytical SEC columns offer high chromatographic resolution and are quick to run.
- No issues regarding over-removal of detergent.
Cons
- This method will work only if your membrane protein sample, and micelles of the detergent in which it is solubilized, have significantly different hydrodynamic radii. If this is not the case, both species will co-elute from the SEC column, leaving you back where you began.
3. Dialysis
“Protein Purification for Dummies” hasn’t been written yet, but when it does hit the shelves, I suspect dialysis will be on page 1. If you’re busy with other experiments or just have a lot of patience, detergent removal by dialysis may be the way to go. [3]
Pros
- The technique is passive, so you can set it up and get on with other work in the meantime.
- Dialysis tubing is very cheap.
Cons
- Not all protein samples tolerate dialysis, so your membrane protein sample may precipitate out of solution.
- It may be hard to find dialysis tubing with a pore size that will permit excess detergent micelles while retaining your membrane protein sample (it is usually the individual detergent molecules that are dialyzed, which is why dialysis is slow).
- It takes a long time to achieve significant separation. It takes even longer if you are using a detergent that has a low CMC.
4. Detergent Removal Columns
There really is a resin for everything these days. Several proprietary technologies now exist to remove excess detergent from membrane protein samples.
For example, HiPPR™ and Pierce™ detergent removal spin columns are both available through ThermoFisher. Your detergent-free sample is just a spin away.
Pros
- Detergent removal columns are relatively inexpensive.
- Very simple to perform, requiring only the columns and microcentrifuge.
Cons
- Lots of plastic waste if you need to need to repeat the detergent removal process.
- It might not work if your membrane protein sample is solubilized in an untested detergent.
- Column capacity is typically low (usually > 100µL), so you may need to concentrate your sample extensively or spin multiple samples at once.
5. Protein Precipitation
Deliberately precipitating your membrane protein sample out of solution probably sounds like a silly thing to do.
If you can refold your protein in vitro, however, then you have an excellent way of removing excess detergent.
Simply precipitate your membrane protein sample, pellet it via centrifugation, and refold it in a sample buffer containing a desirable detergent concentration. [4,5]
Pros
- Requires no specific instruments to perform.
- You get to decide the exact final detergent concentration.
Cons
- It costs both time and sample to discover if you can refold your membrane protein sample in vitro.
- Assaying for activity after you’ve refolded your sample costs even more time.
6. Density Gradient Ultracentrifugation
Fun fact! Ultracentrifuge measurements enabled a PhD student to propose a remarkably accurate structure of DNA in 1948 [6] a full 5 years before James Watson and Francis Crick published the crystal structure of DNA in 1953.
Other nifty applications of the ultracentrifuge include fractionation of cell organelles, [7] determining the size of small particles, [8] and even separating out isotopes of uranium to generate nuclear reactor fuel. [9]
You can also use them to remove excess detergent from membrane protein samples. [10]
Pros
- Can serve as an additional purification step since contaminants that have different relative masses will migrate to different parts of the ultracentrifuge tube.
- Comparatively gentle to your sample compared with other detergent removal techniques.
Cons
- Requires an ultracentrifuge.
- Relevant methods publications cover only a limited range of detergents.
Ways to Remove Excess Detergent from Membrane Proteins Summarized
That’s a lot of information on the different methods of removing excess detergent. To make it easier, here’s a table summarizing the pros and cons of each method.
Table 1. Summary of the pros and cons of the detergent removal methods discussed in this article.
| Detergent Removal Method | Pros | Cons |
|---|---|---|
| Styrene Bead Adsorption | Cheap and simple to perform Beads are available from common chemical vendors | Requires experimental determination of the optimal bead-to-sample Over- or under-removal of detergent if the optimal bead-to-sample is not determined accurately Beads may also remove lipids that are integral to the function of your membrane protein sample |
| Size-Exclusion Chromatography | Uses kit that you probably already have in your lab Impossible to over-remove the detergent | Won’t work if your membrane protein sample and the micelles of your chosen detergent have similar hydrodynamic radii Requires extra downstream processing of your membrane protein sample |
| Dialysis | Cheap and simple to perform Uses kit that you probably already have in your lab | Generally takes a long time to achieve significant separation of detergent micelles from your membrane sample Your membrane protein sample may not tolerate dialysis and precipitate out of solution |
| Detergent Removal Columns | Relatively cheap and simple to perform | Low column capacity means only small volumes of sample can be processed May not work with all detergents |
| Protein Precipitation | Allows you to determine the exact final detergent concentration | Quite a harsh method with no guarantee of being able refold your membrane protein sample in vitro |
| Density Gradient Ultracentrifugation | Gentle to your sample | Requires an ultracentrifuge Not tested on a wide range of detergents |
How to Quantify the Detergent Concentration in Your Membrane Protein Sample
So, you’ve performed your chosen detergent removal method, but how do you determine whether or not the procedure has been successful?
You can get the full lowdown by reading the method paper by Eriks et al. [11]
In summary, iodine vapor stains detergent molecules. You can generate iodine vapor quite simply and safely by gently heating iodine crystals in a vacuum desiccator.
Please do this in a fume hood, however, as iodine vapor is highly toxic . It’s probably a good idea to read the relevant safety data too and have a chat with your safety officer to make doubly sure you aren’t going to hurt anyone.
Behold the pink mist. If you run a regular Thin-Layer Chromatography (TLC) experiment of your membrane protein sample against several detergent solutions of known concentration, you can use iodine vapor to develop your TLC plate.
Laser densitometry will enable you to construct a calibration graph of absorbance against detergent concentration from your developed TLC plate. And using the equation of the graph’s line of best fit, you may then calculate the detergent concentration in your membrane protein sample.
Pro tip! If you don’t have access to a laser densitometer, you can take a high-resolution scan of your TLC plate and use image analysis software such as ImageJ to digitally estimate absorbance density.
Were these tips to remove excess detergent from membrane proteins helpful? Have I left out your favorite detergent removal method? Add it in the comments below if so.
References
- Rigaud JL et al. (1998) Detergent removal by non-polar polystyrene beads. Eur Biophys J 27:305–19
- Allen TM et al. (1980) Detergent removal during membrane reconstitution. Biochim Biophys Acta 601:328–42
- Seddon AM et al. (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 1666:105–17
- Jiang L et al. (2004) Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J Chromatogr A 1023:317–20
- Doucette AA et al. (2014) Resolubilization of precipitated intact membrane proteins with cold formic acid for analysis by mass spectrometry. J Proteome Res 13:6001–12
- Harding SE et al. (2018) The discovery of hydrogen bonds in DNA and a re-evaluation of the 1948 Creeth two-chain model for its structure. Biochem Soc Trans 46: 1171–82
- Alberts B et al. (2002) Fractionation of cells. In: Molecular biology of the cell, 4th edition. Garland Science: New York
- Carney R et al. (2011) Determination of nanoparticle size distribution together with density or molecular weight by 2D analytical ultracentrifugation. Nat Commun 2:335–42
- Theyse FH (1971) The ultracentrifuge separation of heavy isotopes. J Mech Eng Sci 13:168–72
- Hauer F et al. (2015) GraDeR: Membrane protein complex preparation for single-particle cryo-EM. Structure 23:1769–75
- Eriks LR et al. (2003) A strategy for identification and quantification of detergents frequently used in the purification of membrane proteins. Anal Biochem 323:234–41







