If you have sorted samples or phenotyped cells by surface expression of proteins, you’ve probably wondered how each cell is sorted or phenotyped in a flow cytometer? This question seems trivial, but in reality it took a while for engineers to figure it out. Before I get into today’s topic on “hydrodynamic focusing,” I’ll walk you through the mechanical layout of a prototypical flow cytometer. Once you understand how your samples go from point A to Z, you will understand the importance of hydrodynamic focusing.
Flow Cytometry 101
Figure 1 shows how your samples travel from one end of the nozzle (cell intake) to the waste container at the other end (if you are not sorting them).
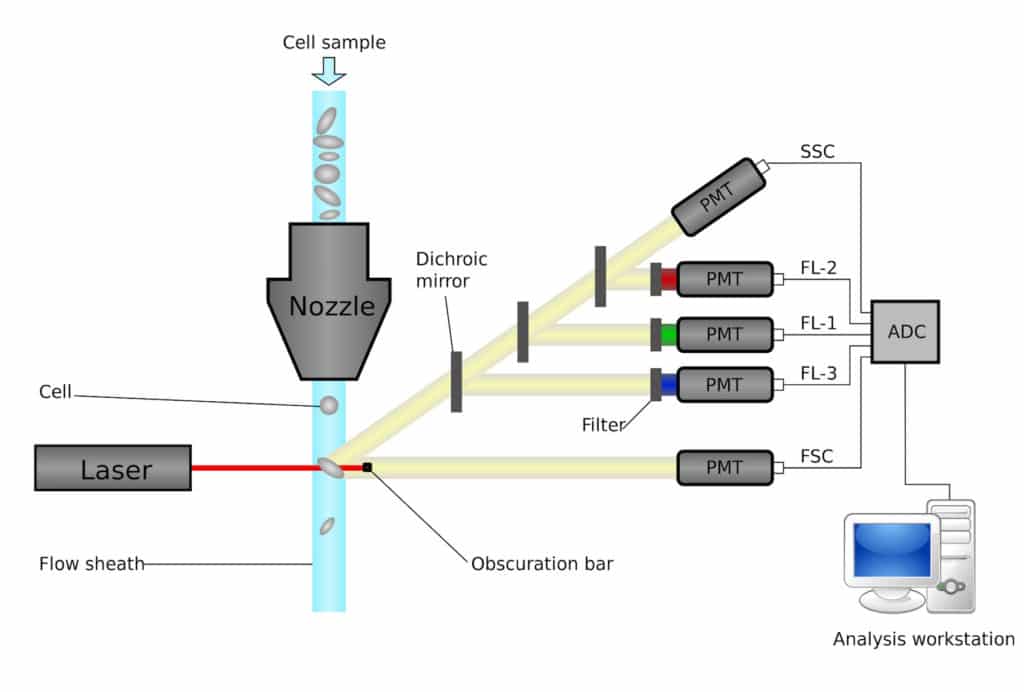
The cells are “sucked up” because of the pressure differences and travel to a crucial integration region called “flow cell,” which is where the magic starts. The cells are “single-lined” in the flow cell (referred to as “nozzle” in figure 1), so different lasers can excite the fluorochromes on the monoclonal antibodies. Additional features, such as cell size and cell complexity, can be verified at the same time. From there, a cell’s “electronic profile” is converted to digital format and stored on the hard drive.
So how does the flow cell make sure that all the cells lineup in a single-file line and follow the queue? We can’t possibly create a path that is narrow enough for only one cell to pass through at a time because the average size of a lymphocyte is roughly 10 to 20 µM in diameter. Believe me on this. If you have ever used a flow cytometer, you know that there are already enough problems trying to unclog samples.
Enjoying this article? Get hard-won lab wisdom like this delivered to your inbox 3x a week.

Join over 65,000 fellow researchers saving time, reducing stress, and seeing their experiments succeed. Unsubscribe anytime.
Next issue goes out tomorrow; don’t miss it.
What other ways can we use to make cells queue up properly? This is where “hydrodynamic” focusing comes into play.
Hydrodynamic Focusing
The true physics behind hydrodynamic focusing are complicated with mathematical calculations and assumptions that I will not get into here. Luckily, you don’t need all that detail to harness the power of hydrodynamic focusing. You just need to understand how it applies in this situation. Basically, when two streams of fluids with different flow rates are running side-by-side and in the same direction into a flow cell, then a laminar flow is created. The central stream (sample stream) is focused and surrounded by the secondary slower stream (sheath fluid). The shape and size of the flow cell is crucial to hydrodynamic focusing, and traditionally the cell is nozzle shaped. However, flow cell designers could also engineer microscopic grooves alongside various locations1 of the flow-cell nozzle to direct the sheath fluid to surround the focusing stream. Pretty neat physics, eh?
In a flow cytometer, the sheath fluid pressure is constant while the sample fluid is adjusted. Therefore, by manipulating the pressure differences, you can get the desired cross-sectional area (i.e., the diameter of a cell). Hydrodynamic focusing properly aligns your cells, one by one, at the junction where the analysis by lasers begins.
Without this step, flow cytometry would not achieve single-cell resolution. For this reason, don’t suddenly change the flow rate because that would change the resolution of the data. Along the same lines, a mechanical failure in the pump that delivers a consistent pressure would result in similar problems. Modern flow cytometers are usually equipped with two pumps to ensure a consistent pressure and flow rate.
New Technology: Acoustic-Assist Focusing
Modern flow cytometers handle thousands of events per second. However, when you increase the flow rate, you lower the overall resolution. How do you solve this problem? Recently, scientists have come to the rescue with acoustic-assist focusing.2 They use sound energy to “usher” the particles into the center of the sheath fluid, where hydrodynamic focusing takes place. This allows high resolution at a high flow rate. In addition, this technology also enables the possibility of running multiple streams at the same time in one flow cytometer.2 This could tremendously speed up the acquisition time!
Hydrodynamic focusing is fundamental to flow cytometry and whoever is running a flow cytometry experiment should understand the principle. Through understanding the principles of hydrodynamic focusing, you can make better judgements on how to fine tune the machine and, therefore, better at running a flow cytometer. And, not least of all, you can always impress your peers with a nice story, too.
If you have any questions, please don’t hesitate to comment below!
References
- Golden JP et. al. 2012. Hydrodynamic focusing – a versatile tool. Anal and Bioanal Chem. 402: 325–35. doi:10.1007/s00216-011-5415-3.
- Piyasena ME et. al. 2012. Multinode acoustic focusing for parallel flow cytometry. Anal. Chem. 84: 1831–39. doi:10.1021/ac200963n.
You made it to the end—nice work! If you’re the kind of scientist who likes figuring things out without wasting half a day on trial and error, you’ll love our newsletter. Get 3 quick reads a week, packed with hard-won lab wisdom. Join FREE here.





