In the first part of this article series, we went through how Photoactivated Localization Microscopy (PALM) works. Now that you have a good understanding of the technique, it is time to start thinking about how to perform good PALM sample prep. In this article, we will cover how to choose which fluorescent proteins to use for PALM, and then go over some tips and tricks for creating the perfect sample.
Fluorescent Proteins – Why Are They Useful, and Which Should I Use?
One of the advantages of using photoswitchable fluorescent proteins is their small size. They are only around 2 nm which means they will likely not interfere with the function of the protein you are interested in imaging. A small size means that a higher labeling density can be reached, and thus a higher resolution achieved.
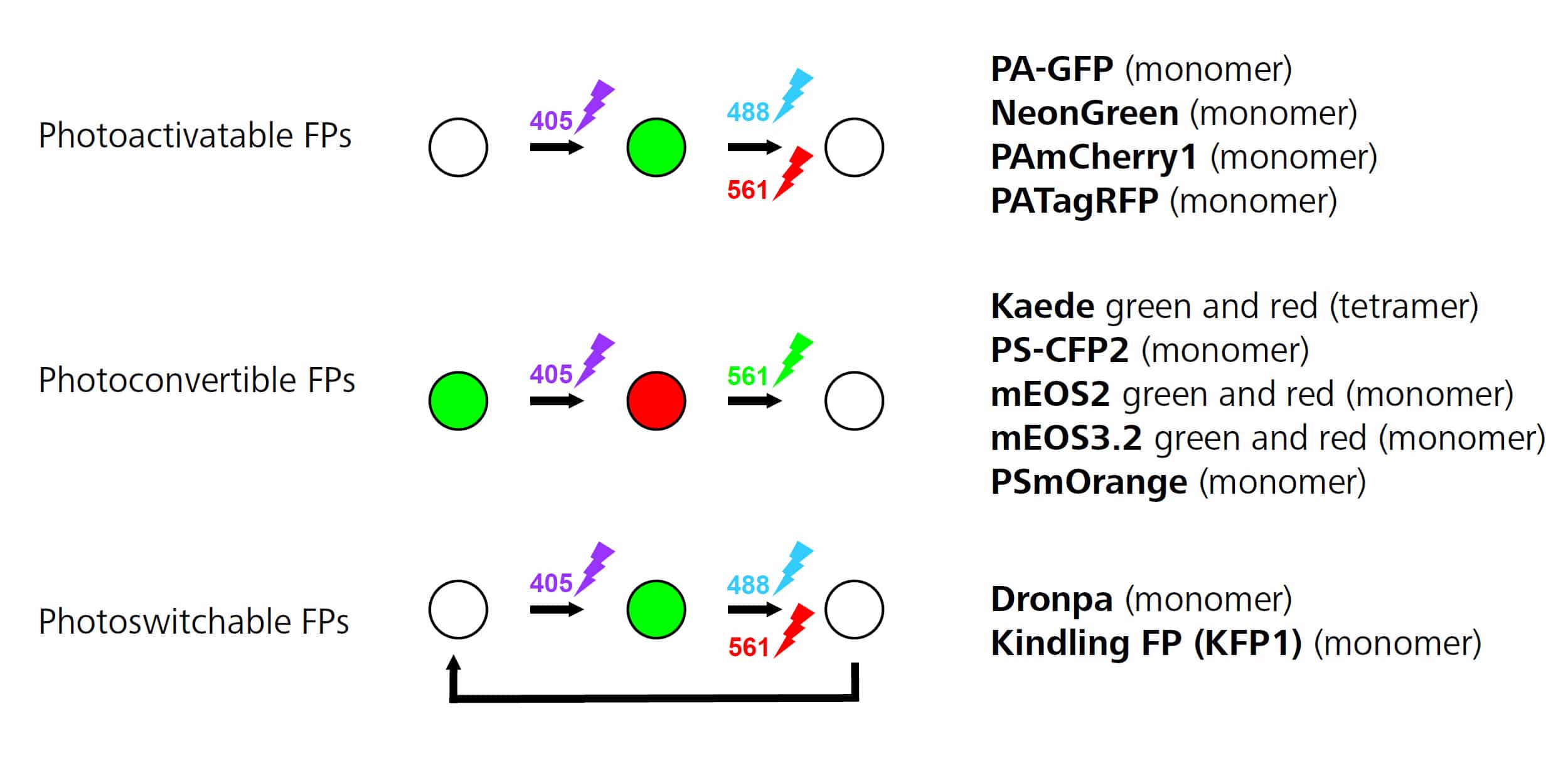
Figure 1. Reproduced from ZEISS ELYRA Sample Preparation guide.
There are different types of photoswitchable fluorescent proteins, as you can see in Figure 1:
- Photoactivable fluorescent proteins are initially not fluorescent and then start fluorescing once they have been exposed to UV light. Switching is irreversible.
- Photoconvertible fluorescent proteins are initially fluorescent at one wavelength (e.g. green) and then change to a different wavelength (e.g. red) after exposure to UV light.
- Reversible photoswitchable or photochromic proteins can be repeatedly switched between a non-fluorescent and a fluorescent state where switching is induced by UV and visible light, respectively.
Photoconvertible fluorescent proteins are the easiest to use because they allow you to visualize the protein or structure you are staining before you begin PALM imaging. This allows you to check that your sample is nice and bright (meaning that your transfection is working well) and that the staining is specific. Due to this, photoconvertible fluorescent proteins such as tdEOS or its monomeric variant mEOS are the most commonly used in the literature. There are a wide variety of fluorescent proteins available in a range of colors,1 and there have been several advances in creating fluorescent proteins suitable for high-resolution techniques such as PALM.2,3
Two-color PALM Imaging
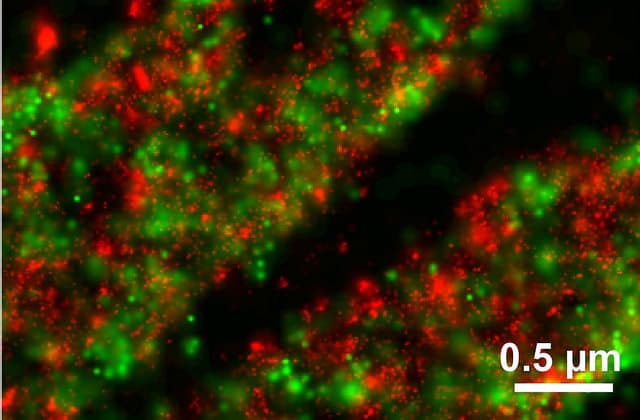
Figure 2. DDronpa fusion of Paxillin (Pax; red) tdEOS fusion of Vinculin (Vin; green). Courtesy of H. Shroff and H. Hess.
Interested in performing two-color PALM imaging? Certain combinations of fluorescent proteins are recommended for this including:
- mEOS2 (\lambdaem ~584 nm) and Dronpa (\lambdaem ~518 nm)
- NeonGreen (\lambdaem ~517 nm) and PA-mCherry1 (\lambdaem ~595 nm)
- Padron (\lambdaem ~522 nm) and Dronpa (\lambdaem ~518 nm)*
When doing two-color PALM imaging,3 it is best to image the dye with the higher wavelength first as this won’t cross-talk into lower wavelength channels. *Despite Padron and Dronpa having similar emission spectrum, they can be used as a pair since they are both reversibly switchable fluorescent proteins, but with opposite directions: i.e. blue light causes Dronpa to switch from fluorescent ‘on’ state to the ‘off’ state, while Padron is switched from fluorescent ‘off’ state to the ‘on’ state when exposed to blue light. Owing to this antagonistic switching, cross talk is negligible and therefore the fluorescent proteins can be imaged in either order.4
PALM Sample Prep: Tips and Tricks
So you have worked out what fluorescent protein to use and are now ready to prepare your PALM sample! Here are a couple of useful PALM sample prep tips to help you on your way:
- Use glass bottom dishes with a coverglass thickness of no. 1.5 (for example – eight-well chambered LabTeks from Nunc).
- Check your fixative. When imaging cells at the single-molecule level, it is vital to make sure your fixative method is properly fixing individual proteins. If PFA isn’t properly fixing your protein of interest, you should be able to see this in TIRF mode. If you are interested in learning more, check out Tanaka et al. 2010.5
- Fresh samples work best.
- If you are not getting a ‘high molecular density per frame’ (number of molecules switched on each frame) then it might be due to your transfection efficiency. Make sure you can get a good enough expression of your transfected protein before you start imaging. Perhaps consider using photoconvertible fluorescent proteins so you can get an idea of your transfection efficiency before you start imaging.
So hopefully this article has given you a good overview of PALM sample prep. It is clear that this Nobel-winning technique has opened up a whole new world of potential biological discoveries – so what are you waiting for… start imaging!
Do you have any other PALM sample prep tips and tricks? Leave a comment below.
Originally published on February 19, 2015. Reviewed and updated on December 17, 2020.
References:
- Kremers GJ, Gilbert SG, Cranfill PJ, Davidson MW, Piston DW. (2011) Fluorescent proteins at a glance. J Cell Sci. 15;124:157–60.
- Patterson GH. (2011) Highlights of the optical highlighter fluorescent proteins. J Microsc. 243:1–7.
- Shroff H, White H, Betzig E. (2013) Photoactivated Localization Microscopy (PALM) of adhesion complexes. Curr Protoc Cell Biol. Chapter 4:Unit4.21.
- Andresen M, Stiel AC, Fölling J, Wenzel D, Schönle A, Egner A, et al. (2008) Photoswitchable fluorescent proteins enable monochromatic multilabel imaging and dual color fluorescence nanoscopy. Nat Biotechnol. 26:1035–40.
- Tanaka KA, Suzuki KG, Shirai YM, Shibutani ST, Miyahara MS, Tsuboi H, et al. (2010) Membrane molecules mobile even after chemical fixation. Nat Methods 7:865-6.