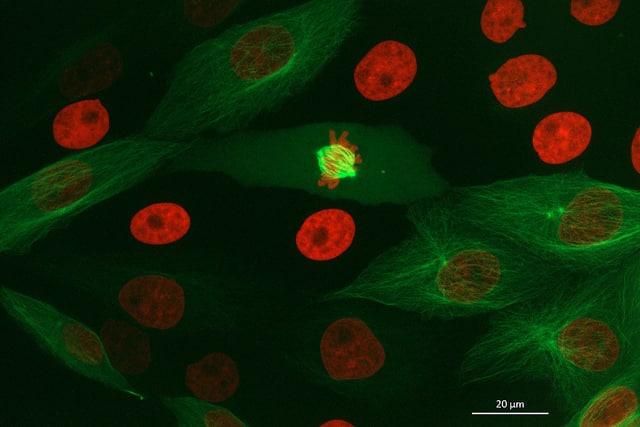
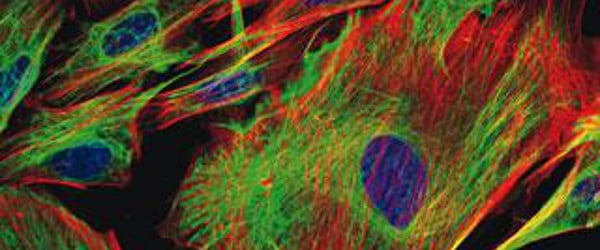
If you remember from one of my previous articles (if not, you can read it here!), we introduced ‘fluorophores’. These are basically substances (natural or synthetic) which have the ability to absorb light at a low wavelength and re-emit at a higher wavelength. In other words- they fluoresce!
In this article, I’ll introduce the three main types of fluorophores which are available to the researcher for fluorescent imaging: Fluorescent proteins, fluorescent probes and quantum dots.
Glowing jellyfish
The history of fluorescence dates back to around 1560, and the history of bioluminescence also stretches back for many centuries. However, it wasn’t until 1955 that the fluorescent component of a living organism was described. This was Green Fluorescent Protein (GFP) from the jellyfish Aequorea victoria and was described in this paper by Davenport and Nicol. It was a further seven years until this component was recognised as a protein by Osamu Shimomura.
Years of cloning
There then followed years of isolation, cloning and expression of GFP resulting in a fluorophore which was of a more practical use by the research community. In 1996, a group led by Roger Tsein reported a single point mutation of GFP which not only increased the usability (in terms of photostability), but resulted in the (now classic) excitation and emission peaks of 488 nm/509 nm. In 2008, the Nobel Prize for Chemistry was jointly awarded to Shimomura, Tsein and Chalfie for their work on GFP (Chalfie first reported the gene encoding GFP).
Many flavors of green
Today, we now have many enhancements and variations of GFP- not all of which are green. GFP now comes in ‘flavors’ including cyan, yellow and blue. Fluorescent proteins are useful for studying live cells and can be used as ‘reporters’ for studying gene expression. Using genetically modified plasmid and/or viral DNA, the target cells can be transfected with the plasmid which encodes both the fluorescent protein and a gene of interest. This localises to the site or organelle of interest within the cell and using fluorescent imaging, this technique can be used to study biological processes over time in living cells.
Covering the spectrum
In essence, fluorescent probes are usually conjugated antibodies which are labelled (or ‘tagged’) with fluorophores. The two most common fluorophores used were FITC (fluorescein isothiocyanate) and TRITC (tetramethyl rhodamine isothiocyanate). However, the range of fluorescent probes (whether conjugated to an antibody, or unconjugated) which is now available is extensive to say the least. One of the most widely used ranges is the ‘AlexaFluor’ dyes from Life Technologies. There are currently 20 AlexaFluor dyes which span the excitation spectrum from 346 nm to 784 nm and are available as labelling kits, or conjugated to primary or secondary antibodies. Thermo Scientific produce their own probes called ‘DyLight’. The ten probes which are currently available cover a spectrum from 350 nm to 800 nm. These are available as the dyes themselves, or as labelling kits. If it’s fluorescent secondary antibodies which you require, then R&D Systems offer their ‘NorthernLights’ secondary antibodies and streptavidin conjugates. Including the main primary antibody raised species, these secondary antibody probes are available in the three main wavelengths used by many labs- those covering the FITC, Rhodamine and Cy5 bands.
I’m pink therefore I’m big!
Quantum dots were discovered in the 1980’s by Louis Brus who was working at the AT&T Bell Laboratories where he was studying the behaviour of semiconductor particles with large surface areas. He made the unexpected discovery that the properties of these semiconductor nanomaterials (or quantum dots) related to their physical characteristics. We’ll take an in-depth look at quantum dots in a later article, but for just now, you just need to know that it is their size which determines their wavelength. For example, as the size of a quantum dot decreases, the wavelength of emitted light increases. It is reported that quantum dots are around 100 times more stable and 20 times brighter than traditional fluorophores. As with other fluorophores, quantum dots can be conjugated to proteins of interest, but as they have a huge surface area, multiple conjugations to a single quantum dot are possible. Companies such as Life Technologies offer ‘Qdots’ as part of their Molecular Probe range.
We’d like to know what you use in your lab- does anyone routinely use quantum dots for imaging? Do you favour DyLight over AlexaFlour? We’d love to hear your thoughts!