Leica Microsystems is a world leader in providing innovative microscopy, camera and software solutions for imaging and analysis of macro-, micro- and nanostructures.
Fluorescence 101: A Beginners Guide to Excitation/Emission, Stokes Shift, Jablonski and More!
Content sponsored by Leica Microsystems

You may already use fluorescence as a tool in your microscopy and imaging work, but, do you know exactly what it is? Why are certain proteins and probes fluorescent? What causes this light emitting property? We’ll have a look at these and more questions in this article.
Start with a definition
We’ll start with a definition of what fluorescence actually is. According to a number of online and printed dictionaries, it’s something along the lines of;
“The visible or invisible radiation produced from certain substances as a result of incident radiation of a shorter wavelength such as X-rays or ultraviolet light.”
In the above statement, ‘radiation’ just means light. We’ll be dealing with light which we can view and image using the microscope- this can include visible, infra-red and ultraviolet.
You’ll also come across the word ‘fluorophore’ when dealing with this subject area. A fluorophore is basically the ‘certain substance’ alluded to in the above statement. These fluorophores re-emit light after exposure to a light particle (or photon). In a later article, I’ll be looking at the different types of fluorophores including fluorescent proteins and fluorescent probes.
Let’s get excited!
Let’s look firstly at that ‘radiation of a shorter wavelength’. When you are dealing with fluorophores, you’ll see reference to ‘excitation/emission’ wavelengths. The ‘shorter wavelength’ light is that which is used as the ‘excitation’ light for fluorophores. The unit of wavelength is the nanometre (nm). You’ll probably come across excitation/emission wavelengths with the lambda symbol and a subscript ‘ex’ or ‘em’ (e.g. ? ex). The shorter wavelength light is absorbed by an electron of the fluorophore and as a consequence, this higher energy photon ‘excites’ the fluorophore.
…and back to ground
This excitation doesn’t last long though as the natural state of the fluorophore is the ‘ground’ state. In returning to this ground state, the fluorophore emits a photon at a longer wavelength (lower energy) and returns once more to a relaxed state. In the fluorophores which you will use for microscopy and imaging, all of this typically happens in a time period of around 0.5 to 20 nanoseconds! This cycle will continue (assuming a continued exposure to the excitation light) until ‘photobleaching’ occurs (see Jen Redig’s explanatory article here).
We can see you
Because the emitted photon is of a longer wavelength than the excitation light, then this difference can be distinguished and detected in the microscope set-up. Furthermore, as each fluorophore has, on the whole, distinct excitation and emission wavelengths, then they can be used to distinguish different targets of interest within the same sample.
Two other terms which are useful to know when setting out to do fluorescence microscopy are Jablonski and Stokes Shift.
Jablonski Diagram
Professor Alexander Jablonski (1898-1980) was a Polish physicist who, in 1933, first illustrated the absorption and emission of light by fluorophores in his now famous diagram. This beautifully simple diagram illustrates the activation from ground state to excited state and the emission of a photon on return to ground state once more. Here’s a simplified Jablonski Diagram:
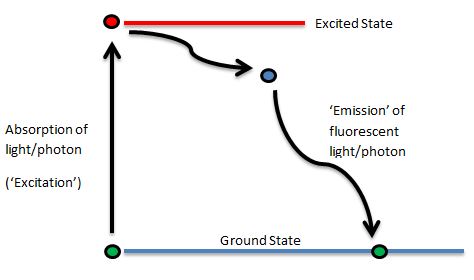
There is not a direct return to ground state as the flurophore can pass through ‘Triplet States’ of energy (additionally, there can be many different ground and excited states), however, we’ll keep it simple just now!
Stokes Shift
Sir George Gabriel Stokes (1819-1903) was an Irish physicist, mathematician and politician. He made many advacnes and discoveries in the fields of optics, light and chemistry (to name but a few!). His work on fluorescence was first published in 1852- you can download the original paper here (entitled ‘On the Change of Refrangibility of Light’). The Stokes Shift (which was named in his honour) is the difference, in nanometres, between the peak excitation and the peak emission wavelengths. Each fluorophore has a distinct and individual Stokes Shift.
That’s all on the basics of fluorescence just now, but please let us knows if there are any topics within this field which you would like us to cover. Keep an eye open for a forthcoming article on the different fluorophores which are available for use in microscopy and imaging.
2 Comments
Leave a Comment
You must be logged in to post a comment.

Hi Richard,
Thanks for the additional info! I kept the history part relatively brief, but would like to (one day!) explore the whole history of fluorescence going back to 1560 (as far as I know),
Cheers,
Martin
John Herschel (1845)made the first observation of fluorescence from quinine sulfate – he called it “epipolic dispersion”. Stokes coined the term “fluorescence” since he did not like the other terms used.