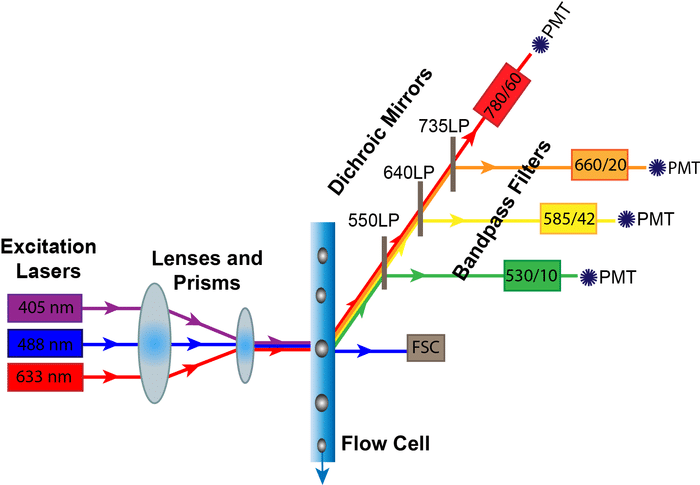
Apoptosis, often called programmed cell death, is a carefully regulated process that is part of normal development and homeostasis. Apoptosis is morphologically and biochemically distinct from necrosis, which is conversely called accidental cell death. Dysregulation of apoptosis is implicated in disease states such as cancer, autoimmune disease and degenerative conditions.
Apoptosis consists of an orderly sequence of events characterized by cell shrinkage, increased cell permeability, changes in membrane asymmetry, chromatin condensation, DNA fragmentation, and cell blebbing. Finally, the apoptotic cells are removed through phagocytosis with minimal tissue disruption.
No single parameter fully defines cell death in all systems, so it is advantageous to use several different apoptosis assays when studying apoptosis. The multiparametric nature of flow cytometry allows the measurement of several apoptotic traits in a single sample, making it a powerful tool to study the complexity of cell death.
You can characterize apoptotic events in early, intermediate and late stages; let’s look at some of the most reliable apoptosis assays for routine use.
Mitochondrial Changes in Apoptosis
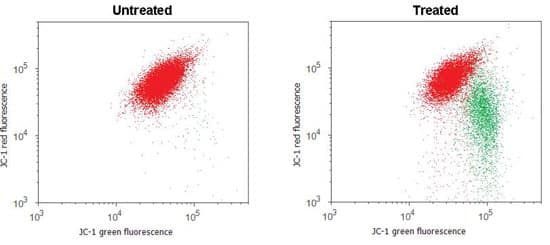
A distinctive feature of the early stages of apoptosis is the collapse of the mitochondrial membrane potential. Probes that mark mitochondrial changes are positively charged, causing them to accumulate inside the mitochondrion, which is negatively charged. JC-1 is a dye widely used in apoptosis studies; it exhibits potential-dependent accumulation in active mitochondria giving red fluorescence with 488 nm excitation. As mitochondrial potential is lost, JC-1 emission shifts from red to green and gives a decrease in the red/green fluorescence intensity ratio. This is shown in Figure 1. The ratio change depends only on the membrane potential and not on other factors like mitochondrial number or size.
DiIC1(5) (1,1’3,3,3’3’-hexamethylindocarbocynanine iodide) is another positively charged dye that accumulates in active mitochondria; it is red fluorescent with 635 nm excitation and shows a decrease of fluorescence intensity with loss of potential. DiIC1(5) is read in the allophycocyanin (APC) channel, making it is easy to multiplex with other reagents.
MitoTracker™ Red CMXRos is another useful mitochondrial probe. It exhibits red fluorescence with 488 nm excitation and shows a decrease of fluorescence as potential is lost. MitoTracker™ Red is read in the phycoerythrin (PE) channel, making it another probe that is easy to multiplex.
Intermediate Events in Apoptosis Trackable by Flow Cytometry
Moving from early to intermediate stages of apoptosis, you can measure the activation of caspase enzymes, changes in cell membrane permeability, and changes in cell membrane asymmetry.
Caspase-dependent Assays
The activation of a series of cytosolic proteases, called caspases, is a hallmark of apoptosis. Caspases are synthesized as inactive pro-caspases; during apoptosis pro-caspases are activated by proteolysis. Several fluorogenic assays for caspase activity are available, including the PhiPhiLux system,and the CellEvent™ Caspase 3/7 Green and NucView 488 Caspase substrates. These cell-permeable substrates are basically non-fluorescent until caspase cleavage.
FLICA (Fluorescent Inhibition of Caspases) is also cell permeant and reacts covalently with a caspase-selective amino acid sequence with a fluorescent tag. Unbound FLICA diffuses out of the cell and is washed away.
Membrane Permeability Assays
Three monomeric cyanine dyes, PO-PRO™-1, YO-PRO®-1, and TO-PRO®-3, enter apoptotic cells because of increased cell membrane permeability. These dyes bind to nucleic acids once inside the cell, while cell-impermeant dead cell stains, such as propidium iodide are excluded. The three aforementioned dyes have different excitation wavelengths, 405 nm, 488 nm and 635 nm respectively, providing flexibility for multiplexing.
Membrane Symmetry Assays
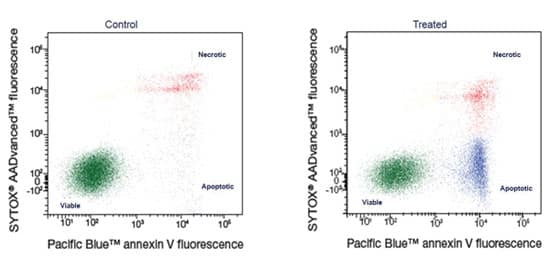
Loss of cell membrane asymmetry occurs as apoptosis progresses. Phosphatidylserine (PS) is located on the inner leaflet of the cell membrane in live cells. As apoptosis progresses, PS translocates, or flips, to the outer leaflet of the membrane. Annexin V labeled with a fluorophore identifies apoptotic cells by binding to PS on the outer leaflet. When paired with an impermeant dead cell stain, you can distinguish live, apoptotic and necrotic cell populations, as seen in Figure 2. Annexin V binding is dependent on the presence of calcium and magnesium in your buffer, so be sure to include both at all stages of Annexin V testing.
The Violet Ratiometric Membrane Asymmetry Probe, F2N12S, is a novel 405 nm excitable dye that detects variations in surface charge associated with PS flipping. Similarly to JC-1, F2N12S gives two emission bands; viable cells produce an orange emission which shifts to green with apoptosis, and the ratio of orange/green emission decreases.
Flow Cytometric Analysis Nearing the End of Apoptosis
The later stages of apoptosis involve nuclear changes, including chromatin condensation and DNA fragmentation.
Morphologically, the nuclei of apoptotic cells become smaller than those of normal cells and display higher fluorescence when labeled with UV excited Hoechst 33342 or 405 nm excited Vybrant® DyeCycle™ Violet stains. When paired with an impermeant dead cell stain, you can distinguish live, apoptotic and necrotic cell populations with these Chromatin Condensation Assays.
DNA fragmentation in apoptosis can also be examined using the TUNEL assay (terminal deoxynucleotidyl transferase-dUTP nick end labeling). The assay is based on the incorporation of modified dUTPs by terminal deoxynucleotidyl transferase (TdT) at the ends of fragmented DNA. A new TUNEL kit uses click chemistry to incorporate an alkyne-modified dUTP at the ends of fragmented DNA using TdT, and then detects it using a copper-catalyzed click reaction with an azide labeled fluorophore.
Tips for Successful Apoptosis Assays by Flow Cytometry
To get the best results in your apoptosis assays, use the following tips:
- It’s always a good idea to include controls, both viable and apoptotic, especially when using a new assay, cell type, or stimulus.
- During sample preparation, treat your cells gently to avoid induction of apoptosis through manipulation.
- To avoid post-assay apoptosis, analyze cells as soon as possible after labeling.
- Background fluorescence of viable cells will generally have higher background fluorescence than unlabeled cells.
- Be aware that flow cytometric analysis of apoptosis in adherent cells presents unique challenges, as methods for cell removal may trigger false apoptotic events.
- Use caution when combining immunophenotyping with apoptotic assays; antibodies can non-specifically bind to dead cells, making interpretation difficult. Use the earliest indicator of apoptosis you can in this case.
- Finally, it is always a good idea to confirm morphology whenever possible. Apoptosis is a highly variable process; no universal morphological or physiological characteristic is common to all cell types; looking at multiple parameters of apoptosis is recommended.