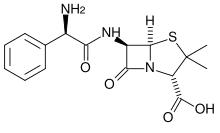
How Ampicillin Selection Works
The basis of ampicillin selection is the hydrolysis and inactivation of the antibiotic by beta-lactamase expressed from the plasmid-borne bla gene. Here’s the problem: beta-lactamase, which is produced at substantial levels, is also secreted by the bacteria into the medium. The resulting build-up of extracellular beta-lactamase can inactivate the ampicillin in the culture medium, removing the selective pressure, if the culture is not handled properly.
In liquid cultures, this means that a portion (possibly a very large portion) of the cells may no longer have the plasmid, giving poor yielding plasmid preps, protein expression etc. On agar plates, ampicillin degradation can lead to the formation of satellite colonies. These are very small colonies of cells that have not taken up the plasmid that form around a large colony that has taken up the bla-containing plasmid. The satellites form because the beta-lactamase released by the bla-expressing colony degrades the ampicillin in the vicinity of the colony. The satellites are not necessarily a problem as they will not grow when transferred to a medium containing fresh ampicillin.
Avoiding plasmid loss
If you are noticing poor yields in your plasmid preps or less than desirable protein expression, beta-lactamase build up may be your issue. So how do you avoid plasmid loss when using ampicillin as a selection marker? Here’s how:
- Don’t allow liquid cultures to saturate for too long. I would recommend never growing cultures higher than OD600= 3 (in lysogeny broth, also know as LB).
- Remove secreted beta-lactamase from the starter cultures. This can be done by pelleting and re-suspending the starter culture in fresh, antibiotic-free medium before innoculating the main culture.
- If you are experiencing problems, use a higher ampicillin concentration. I would recommend using 200 ?g/mL or higher. This makes it harder for the beta-lactamase to inactivate all of the ampicillin and is especially useful for avoiding satellite formation.
- Never use old ampicillin stocks or plates as the ampicillin will have broken down somewhat, giving a reduced effective ampicillin concentration. For more information on how to store and use antibiotics read our related article on Antibiotic Stability: Keep Your (Gun)powder Dry.
- If all else fails, switch to carbenicillin selection. While this antibiotic is also inactivated by beta-lactamase, the process occurs much more more slowly than with ampicillin. For this reason carbenicillin selection is far more effective than ampicillin. Unfortunately it is much more expensive!
Ampicillin is just one of a variety of anitbiotics used in molecular biology. If you want information about other commonly used antibiotics, check out our article on Antibiotics Used in Molecular Biology.
Do you have any tips on working with ampicillin? If so, please leave a comment.
And if you’re wondering which antibiotic is just right for your application, download our free antibiotics reference guide poster and pin it up in your lab.
Originally published on May 9, 2011. Revised and updated on December 17, 2019.