Adaptive Focused Acoustics® (AFA®) technology by Covaris is a precisely controlled, mechanical method enabled by acoustic energy operating at a specific frequency used for efficient lysis of cells.
Extraction of cellular analytes (such as nucleic acids, proteins, and metabolites) using AFA consistently offers high yield and quality, thereby making this technology well-suited for many workflows, from Next Generation Sequencing (NGS) to multi-omics.
In our previous articles, we’ve looked at the benefits of AFA based workflows for cell lysis compared to other traditional methods and highlighted examples of how AFA enabled workflows improve the yield, depth and coverage of proteins and peptides from a host of different matrices.
This article will focus on hands-on execution of AFA-based workflows for protein extraction from different matrices for reliable, reproducible downstream proteomic analysis.
Optimizing Protein Extraction for Quality and Yield
To bring you the best practical advice on using AFA-based workflows for protein analysis, we interviewed Sameer Vasantgadkar, Senior Manager for Omics Solutions at Covaris.
We sought his advice on how to optimize focused-ultrasonication settings to maximize protein yield, coverage, and peptide depth for a wide variety of sample types. In addition, Sameer also shares several useful workflow tips for efficient extraction of proteins from cells, fresh-frozen (FF) tissues, and formalin-fixed paraffin-embedded (FFPE) tissue samples.
We started by asking Sameer what combination of ultrasonicator settings usually gives the best protein coverage and yield.
“Proteomic workflows can have a lot of different processing combinations across different sample types, buffer types, and processing volumes. Covaris’ AFA-enabled workflows are uniquely designed to address all these requirements.”
He explained that there are five instrument parameters you can adjust and optimize based on the sample matrices and starting volume.
In the table below, we’ve summarized these parameters.
Table 1. Ultrasonicator instrument parameters with corresponding scenarios.
Instrument Parameter | Description | Scenario |
Peak Incident Power (PIP) (W) | The peak electrical power used to drive the ultrasound transducer. It represents the amplitude of the sonic wave. | Higher PIP means higher power. Tough samples (hard tissues, organoids, bacterial cells, etc.) may need a higher PIP setting. |
Duty Factor (DF) or Duty Cycle (%) | Controls energy delivery. It is the extent to which the transducer stays on in a given sonic burst. It is expressed as a percentage of the total operation time. | Higher DF means more power has been delivered. Viscous or difficult to homogenize samples will need a medium to higher DF setting. |
Cycles per burst (CPB) (#) | The number of electrical signals, or oscillations, happening in a given sonic burst. | Higher CPB results in a higher degree of mixing. High CPB is required for tough or extremely viscous samples to ensure proper homogenization. Low CPB can be applied for low-volume samples for proper homogenization while minimizing the extent of sample splashing. |
Duration | Total amount of treatment time. | In general, a longer treatment time is used for more thorough processing. Time can be increased if you have maxed out the power and still need more homogenization. |
Temperature (°C) | Temperature inside the consumable. | <10 °C for native protein extraction. 20 °C for protein extraction with detergent based buffers, e.g., SDS detergent. 37 °C for trypsin digestion. |
The number of settings and sample combinations can be quite large. While it is difficult to have specific settings for each possible combination, Sameer emphasized that optimal experimental conditions have been optimized for many samples and are provided in their Quick Guides for protein analysis sample preparation.
“We are always available and happy to help with customization of parameters for the specific samples. However, we strongly recommend starting with the settings that are reflected in our Quick Guides. These settings have been tested both internally and by our users across different processing volumes for a variety of sample types.”
Optimization Beyond the Quick Guides: Try a Peak Incident Power Course
Although the Quick Guides are extremely comprehensive, we wanted to find out if there is any systematic way users can quickly determine an optimal combination of parameters for any sample types that are not included in this guide.
“Proteomics samples can vary based on their types and volumes. If you’re working with any samples, or if you want to consider a different setting configuration that is not covered in the Quick Guide, a good way to optimize your settings is by doing what we call a PIP course and a time course, basically trying with a range of power and time courses.”
“We recommend taking three different PIP settings (or power settings) and three time settings to map out the operational space to see the combination that offers the best performance. The best performance can be determined in different ways: for protein extraction conditions, you can perform a quick bicinchoninic acid (BCA) assay post-extraction to determine the total protein concentration. This is how you can figure out the sweet spot for the best performance, be it with your volume, DF, CPB, or a combination of all of them.”
With clear instructions on how to tune AFA parameters, we asked Sameer for practical tips on working with the following sample types:
- Cells
- FFPE Tissue
- Fresh Frozen (FF) Tissue
Tips for Working with Cells
Troubleshooting Issues with Sample Transfer
A common issue during cell processing is that resuspending cells in lysis buffer can make the sample too viscous to transfer into the Covaris consumable. Figure 1 shows examples of some of the commonly used Covaris consumables for cell lysis.
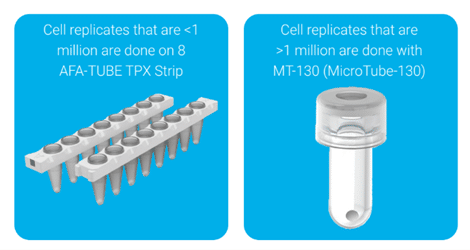
Figure 1. The Covaris 8 AFA-TUBE TPX strip (left) is compatible with cell numbers less than 1 million, and the Covaris microTUBE-130 Bead Snap-Cap (right) is compatible with samples of greater than 1 million cells.
Loss of samples or challenge during sample transfer occurs because the detergent in the lysis buffer breaks open the cells, releasing DNA, which in turn, significantly increases sample viscosity.
According to Sameer, there are two ways to manage this issue:
- Resuspend the cells in a simple buffer such as Phosphate-buffered saline (PBS), followed by gently transferring them to the Covaris consumable with approximately half of the processing volume. This step should be followed by the addition of detergent or 2x strength lysis buffer to achieve the final concentration.
- Collect the cells in a simple buffer such as PBS, transfer them to the Covaris consumable, centrifuge them, discard the supernatant, and resuspend them in lysis buffer. Even if the cells lyse during this process, the extraction efficiency will not be compromised, as the sample is already in the consumable you will expose to AFA. This approach is particularly beneficial for scenarios where you cannot spike in the detergent.
Achieving High-throughput Processing of Adherent Cells
Adherent cells require a surface to attach to and grow on, forming a monolayer in a culture. They are commonly used in research for studying cell behavior, drug testing, etc. Increasing the throughput of workflows with adherent cells is challenging due to the lack of automated methods to detach cells.
Traditionally, you detach the cells using trypsin or manual scraping, which are not only laborious but also can be time-consuming.
In this regard, Sameer explained how Covaris’ protein analysis sample preparation workflow with the AFA technology can detach cells without scraping or trypsin.
“We have tested this in-house, and some of our partners are now using it. When you’re working with adherent cells, certain instrument models can use AFA technology to detach the adherent cells from the cell culture plate without scraping or adding trypsin. In this assay, you would use a very mild AFA energy, which helps in efficiently detaching the adherent cells. They come off as a film, which you can then transfer into the appropriate consumable for lysis.”
This approach has proven beneficial for researchers who work with adherent cells and want to reduce hands-on time and achieve semi-automation. You can read the protocol on how to do it in the poster below. [1]
Tips for Working with FFPE Tissue
Covaris’ AFA-based workflows have been used for years for FFPE samples to efficiently remove paraffin, rehydrate tissue, and extract high-quality nucleic acids (DNA and RNA) at the same time.
The standardized, temperature-controlled workflow reduces sample variability, minimizes hands-on time, and yields higher quality nucleic acids suitable for downstream applications like NGS.
While the same approach can be adopted for proteomics to deparaffinize the FFPE samples, these samples can still present an array of challenges, ranging from the transfer step to ensuring efficient protein extraction.
Avoiding Static and Contamination
Using a microtome (an instrument for cutting extremely thin sections of material for examination under a microscope) to cut FFPE slices from an FFPE block normally offers clean cuts, but certain FFPE blocks can be flaky, and the resulting scrapings can break apart.
We wanted to understand how researchers could prevent sample loss due to static electricity on scrapings, causing them to stick to the top of the tubes, move around, or even jump into adjacent tubes.
Sameer explained that, when working with Covaris AFA-TUBE TPX plates or strips, you may want to pre-fill wells or tubes with lysis buffer so that you are transferring into liquid, thereby neutralizing the static.
“This was done by one of our collaborators, the Broad Institute, where they showed performance was similar when you collect the samples in dry tubes or with samples in tubes that already contain lysis buffer.” [2]
Not only did the group report that there was no impact on protein yield, but they also found it easier to collect samples into tubes pre-filled with lysis buffer (Figure 2).
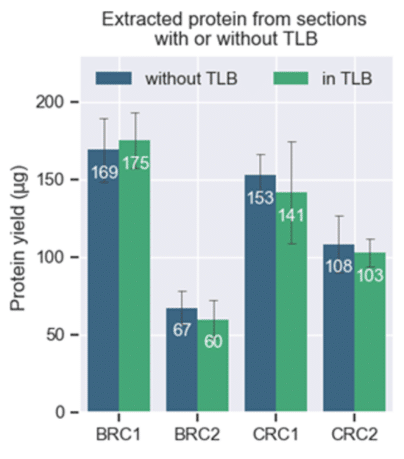
Figure 2. Comparable protein yields from breast cancer (BRC) and colorectal cancer (CRC) sections (from FFPE scrolls) transferred to the Covaris tube with or without tissue lysis buffer (TLB) in the tube. [2]
Sameer also had further advice for dealing with static, including using an anti-static gun when transferring a tissue scroll.
In addition, and in order to make it easier for the user, the recently launched Covaris truPREP® Protein FFPE Kit includes individual tubes for sample collection and extraction. Sameer recommended sealing these tubes as soon as the tissue is transferred to prevent the static from causing tissue to jump between tubes.
Laser Capture Microdissection (LCM) Samples
LCM is a precise technique that uses a laser under a microscope to isolate specific cells or small tissue areas from a heterogeneous sample, such as a tumor, for molecular analysis. However, despite the precise sectioning of tissue samples with LCM, there are several problems specific to working with such samples.
One of the objectives of LCM is to excise a precise sample volume, but FFPE samples that are sectioned using LCM can be tricky to transfer without contamination.
Sameer pointed to the fact that many of Covaris’ partners have had great success with third-party adapters compatible with Covaris plates and strips. These hold the Covaris consumable under the slide and directly transfer samples post the microdissection step. Once transferred, for the LCM samples, you do not require a separate deparaffinization step as the samples are fairly small in size. These LCM samples can be homogenized, followed by extraction of proteins, purification, and digestion using the above-mentioned FFPE workflow.
The part numbers for the commonly used adapters are:
- Leica Holder 11103285 [96-well plate]
- Leica Holder 11505305 [8-well strip]
Tips for Working with Fresh Frozen Tissue Samples
Covaris’ microTUBE-130 Bead Snap Cap (Figure 1, right) enables complete homogenization of multiple samples of fresh frozen tissue (1-10 mg input amount) without any prior cryopulverization step.
For FF tissue sample amounts between 10–50 mg, a cryopulverization step is usually recommended, followed by transferring the sample to the above-mentioned consumable.
Once in the consumable, the homogenization step, followed by purification and digestion, can be carried out using a similar protocol as described above.
Processing Different Samples in Parallel
If you want to process different tissue types (e.g., heart and lung tissue) or use different input amounts within one batch, Sameer recommended performing a PIP course (see above).
This will let you find a single combination of settings that can work for the entire batch of samples.
If you work with varying sample masses within one batch, Sameer suggested that the homogenization step should be carried out for the duration needed to completely homogenize the sample with the largest mass.
For example, five minutes of homogenization may be sufficient for a 2 mg sample, but it may take 15 minutes to homogenize a 10 mg sample. In this scenario, where you might have a combination of 2 mg and 10 mg samples in a given plate, it would be better to process the entire batch for 15 minutes.
You can test to see if your treatment has worked for all samples within the timeframe by:
- Visually inspecting your samples to see if they are all completely homogenized. The microTUBE-130 Bead Snap Cap is made from glass, so it should be clear if anything remains unprocessed.
- Determining the protein yield using a BCA assay (as described above). Typical yields for most tissue types are 70–120 µg/mg. A protein yield in that range is a good indicator that the extraction has worked for all samples.
How to Minimize Processing Time for Tissue Samples
A recurring question that we wanted to ask Sameer is how users can reduce the time needed to process tissue samples.
“In our experience, using disposable biopsy punches facilitates quicker sample trimming and aliquoting than having to use a scalpel to cut the tissue and transfer it. So, we always recommend disposable biopsy punches.”
Sameer also pointed out that some users have found the tissue cutting process easier with the additional help of a dissecting kit. [3]
We also wanted to learn how to save time and sample during the weighing steps.
Sameer explained that if you need to weigh samples to the nearest milligram or if you work with small samples (1–10 mg), reducing the number of transfer steps will minimize unwanted sample loss.
In this regard, the most efficient process will be to measure the mass of the Covaris tube you intend to use and cut your sample directly into it. Then, measure the mass of an empty Eppendorf® tube, and place the Covaris tube, with the sample inside, into the Eppendorf tube.
The mass of your sample will be the difference between the mass of everything and the combined mass of the Covaris tube and the Eppendorf tube.
If you work with significantly larger masses (25–300 mg), Sameer recommended using a Covaris cryoPREP® Dry Pulverizer as the first step for reducing sample pre-processing time.
Preparing Samples for Native Protein Extraction
While many protein studies require denatured samples, sometimes protein structure must be maintained throughout the extraction process. We asked if Sameer had any advice for native protein extraction.
“For native protein extraction, you must use an appropriate protease inhibitor cocktail. In addition, we set up the method so that once you transfer the tissues, you can quickly cycle through each column with partial treatments, thereby ensuring minimal downtime for tissue processing.”
For example, if you are running half a plate and you have six columns to process, you would:
- Run column one for 10 seconds
- Run column two for 10 seconds
- Continue for all six columns
- Once all six columns are partially processed, return to column one
This approach ensures all columns are processed within similar times and that there is no excessive downtime where samples are left idle. This approach has the additional benefit of not heating the samples, which prevents denaturation.
Troubleshooting Common Issues
Incomplete Processing
Very often, samples are not processed or homogenized completely. The incomplete homogenization can be attributed to improper setup of the ultrasonicator. You can mitigate this issue by making sure you are using:
- The correct consumable
- The correct accessory
- The correct method settings
- The correct plate definition
“These four steps can ensure the accuracy of the method, and therefore high quality of sample processing. Sometimes we have seen that either the rack or the plate definition was wrong, so the focal point was off. The plate definition is something that covers the temperature and the focal point. So, if the wrong file is used, it will reduce performance.”
It’s important to note that there are different plate definitions for different consumables, based on their size. The type of rack and plate definition you use impacts the positioning of the focal point.
Unexpected Drops in Performance
If the cause of any unexpected drop-off in performance is not easily identified, you can perform a ‘sanity check’ by looking at the history files. The history files log the conditions used on prior samples and list them by day and time.
If the settings from the latest experiment do not match previous test conditions that gave good results, Sameer recommended reverting the method conditions to resolve the issue.
“Things worked two weeks back. Now it’s not working. You can go back and check the history files and see on that day, at that time point, what conditions were used. Do they match what is being used today?”
In case the parameters match but the issue of inconsistent results persist, send your history and log files to Covaris where a team of technical specialists can investigate the issue for you.
Email applicationsupport@covaris.com or complete this form: Contact Application & Technical Support | Covaris.
Maintenance Checks
We concluded our chat with Sameer by asking about basic instrument maintenance and how often Covaris’ focused ultra-sonicators should be cleaned.
“The maintenance on the Covaris instruments is very simple. Most instruments from Covaris have a water conditioning system which have inline filters. So, water is already circulating through the water conditioning system through those filters, and it’s getting cleaned on a daily basis or whenever the circulation is on.”
He also said users should clean the instrument with a bleach alternative once a month, independent of how often they use it. NaDCC tablets are available at covaris.com.
Summary: Protein Extraction for Proteomics Using AFA
The adaptability of protein sample preparation using AFA technology across all sample types, amounts, and protein types means that you can optimize your workflows in many ways.
Start with the settings mentioned in the Quick Guides, which are tested and validated by a team of experts at Covaris and can be used as a strong starting point for most applications. Alternatively, use a PIP course as a starting point to optimize AFA settings.
Things to check out routinely:
- Covaris’ application notes or the sample-specific tips above to help you design the most efficient AFA-assisted workflow for your sample type.
- Regularly check your Covaris instruments’ history files to ensure you use consumables, method settings, and plate definitions consistently.
- In case of any problems, send your history files to Covaris, where a team of specialists can support developing, optimizing, and executing new workflows or troubleshooting existing ones.
References
- McCarthy P, Muriph R, Beker L, Autret N, Laugharn J, Evans J, and Thomann U. (2022, June 5-9). From Tissues to LC-MS-ready Protein Digests: Automated Sample Preparation Enabled by Adaptive Focused Acoustics® (AFA®) Technology [Conference poster]. American Society for Mass Spectrometry 70th Conference on Mass Spectrometry and Allied Topics (ASMS), Minneapolis, Minnesota, United States
- Haines M, Thorup J, Gohsman S, Heil L, Vasantgadkar S, Abarzua L, Newton C, Rohrer D, Hostetter G, Mani DR, Gillette MA, Carr SA, Satpathy S. (2024, June 2-6). High-throughput, streamlined processing workflow of formalin-fixed paraffin-embedded (FFPE) tissue yielding up to 10,000 proteins per sample [Conference presentation and poster]. American Society for Mass Spectrometry 72nd Conference on Mass Spectrometry and Allied Topics (ASMS) 2024, Anaheim, California, United States
- Ortiz J, Keoseyan A, Bhosale DS, Hendricks N, Seyedmohammad S, Stotland A, Vasantgadkar S, Abarzua L, Moradian A, Mockus S, Van Eyk J. (2024, June 2-6). High-throughput and automated cellular and tissue lysis using COVARIS acoustic technology for proteomics [Conference presentation and poster]. American Society for Mass Spectrometry 72nd Conference on Mass Spectrometry and Allied Topics (ASMS) 2024, Anaheim, California, United States