The in-cell Western assay is a powerful technique that has enhanced how researchers analyze protein expression levels and signaling pathways within fixed cells, allowing scientists to screen multiple samples quickly in a whole-cell environment.
Unlike traditional Western blotting, which involves cell lysis and protein extraction, in-cell Westerns allow researchers to obtain valuable data from targets inside fixed cells with their native microenvironment intact.
This article explains in-cell Westerns, their primary advantages, applications, and some of the best technology and products with which to perform them.
What are In-Cell Westerns?
In-cell Westerns combine the specificity of a regular Western blot with the high-throughput nature of an ELISA.
In-cell Westerns enable measurement of protein expression levels and post-translational modifications inside fixed cells. This makes them extremely useful for studying signaling pathways, protein–protein interactions, and dynamic changes in protein expression.
Although in-cell Westerns are not carried out inside live cells (the cells are fixed and permeabilized), they are much more physiologically relevant than traditional Western blots owing to the lack of cell lysis.
Like a traditional Western blot, target molecules (usually proteins) are detected using antibodies. However, cell fixation and membrane permeabilization enable the delivery of the antibodies into fixed cells for quantification, eliminating the need for time-consuming transfer and electrophoresis steps.
ELISAs also detect proteins using antibodies. In an ELISA, purified target molecules captured from complex biological samples like cell lysates or serum are immobilized in a microplate well and then probed with antibodies. While ELISAs eliminate the need for cell fixation and permeabilization steps, as required for in-cell Westerns, samples are not in their native physiological environment when they are analyzed.
Applications of In-Cell Westerns
The versatility of in-cell Westerns and their comparatively gentle nature make them a valuable tool in various research areas. Their potential applications include:
- Studying cell signaling pathways and protein dynamics in response to stimuli or drug treatments. [1]
- Investigating post-translational modifications, such as phosphorylation and acetylation. [2]
- High-throughput screening for drug candidates. [3]
- Identifying cellular changes in disease models. [4]
Here are three specific examples of how researchers are using in-cell Westerns for biomedical applications:
- Wan Y et al. exploited the high-throughput nature of in-cell Westerns to develop a rapid assay that detects influenza A replication to try and mitigate the medical burden of influenza A pandemics. [5]
- Egorina, Sovershaev, and Østerud used an in-cell Western to quantify Tissue Factor (TF) levels, which is critical to stopping bleeding from a blood vessel but poorly understood because of a lack of suitable techniques for studying resting or lipopolysaccharide-stimulated human mononuclear cells. [6]
- Filardo et al. used in-cell Westerns to develop a high-throughput method to quantify Chlamydia trachomatis and screen anti-chlamydial drugs. [7]
How Do In-Cell Westerns Work?
In-cell Westerns involve five main steps:
1. Cell Seeding and Treatment
Cells of interest are seeded as a monolayer into multi-well plates and may be subjected to different experimental conditions or treatments depending on the experimental goal. The cells should be evenly spaced, viable, and able to proliferate in a multi-well plate.
The cells, which are adhered to the bottom of the plate, are analogous to the immobilized antigens in an ELISA.
2. Fixation
Cells are fixed to preserve their native state and to immobilize the target molecules within the cells. The choice of fixation agent depends on what the target antigen is, how delicate the epitope is, and if the fixation agent is compatible with downstream experiments.
The primary fixative choices are paraformaldehyde, methanol, ethanol, and acetone.
- Paraformaldehyde is ideal for preserving cellular morphology and structural integrity. Use it primarily for immunofluorescence and immunohistochemistry, as it retains antigenicity and allows visualization of cellular components in their native context.
- Methanol is particularly effective for preserving cellular proteins, making it suitable for detecting intracellular antigens. It also permeabilizes cell membranes, facilitating the antibody penetration required for immunofluorescence applications and eliminating the need for separate permeabilization steps.
- Ethanol is good for the rapid fixation and dehydration of cells. It is often combined with other fixatives to make it more effective at preserving antigens. Like methanol, it is also a permeabilization agent.
- Acetone is great for preserving delicate antigens and nucleic acids. It is also relatively fast-acting and achieves good permeabilization for antibody penetration.
Be careful during this step to avoid detaching cells from the plates, as the monolayer of cells at the bottom of the multi-well plate is crucial for a successful in-cell Western.
3. Permeabilization
Cell membranes are permeabilized to allow efficient antibody penetration and access to intracellular proteins.
Common permeabilization agents include Triton X-100™ and TWEEN™ 20. Incubating the cells with these solutions gently disrupts the lipid bilayer of the cell membrane without causing extensive cellular damage or detachment.
As with the previous step, care should be taken to avoid detaching cells from the plates.
4. Blocking, Washing, and Antibody Incubation
Like a traditional Western blot, the cells are washed and blocked to prevent non-specific binding. Blocking agents such as bovine serum albumin (BSA) or non-fat dry milk saturate non-specific binding sites, minimizing non-specific binding of antibodies during subsequent steps.
After blocking, the cells are incubated with primary antibodies that recognize target molecules. Following incubation, there is an additional wash step to remove any unbound primary antibody.
After washing, secondary antibodies labeled with fluorophores are added to amplify the signal and allow detection of the targets. The secondary antibodies are specific to the species of primary antibodies (e.g., anti-mouse or anti-chicken), functionally tagging the targets with fluorescent signal.
Azure Biosystems offers a variety of fluorophore-labeled secondary antibodies that functionally tag target molecules with a fluorescent signal. Browse their selection of secondary antibodies here.
Why Use Near-Infrared Labeled Antibodies?
While visible-spectrum antibodies are compatible with in-cell Westerns, near-infrared antibodies are the preferred species used for detecting target molecules.
Reduced Background Signal
Near-infrared labeled antibodies emit fluorescence in a wavelength range of approximately 700–900 nm, which is distinct from the autofluorescence typically produced by cellular components in the visible region of the electromagnetic spectrum (around 400–700 nm). Using near-infrared wavelengths significantly reduces background fluorescence from the cellular environment, leading to higher signal-to-noise ratios and improved sensitivity.
Available Conjugates
There is a wide range of commercial near-infrared-conjugated secondary antibodies available, making it easy to find suitable antibodies for specific targets. If researchers can’t find the antibodies they need, they can label their own antibodies with near-infrared fluorophores.
For a convenient kit that allows the labeling of any validated primary or secondary antibody to perform in-cell or traditional Westerns in less than an hour, use the AzureSpectra Kit. The labeled antibodies are further compatible with multiple immunofluorescent platforms, including flow cytometry and immunofluorescent microscopy.
5. Detection and Imaging
The final step is to visualize the in-cell Westerns using an infrared imaging system such as the Azure Sapphire™ FL. These images provide quantitative protein expression data of the samples.
Laser scanners measure immunofluorescence from the bottom of a multi-well plate and are, therefore, the ideal choice for imaging in-cell Westerns.
The Azure Biosystems Sapphire™ FL Biomolecular Scanner offers exceptional resolution—down to 5 microns—and features chemiluminescence, fluorescence, and phosphor imaging modes.
Figure 1 provides an example of results from an in-cell Western experiment. The data comes from an experiment performed at the Parreno Lab, University of Delaware. The goal was to quantify protein regulation in chondrocytes (the cells that help maintain cartilage) and test drugs that promote cartilage deposition in cells to potentially treat osteoarthritis.
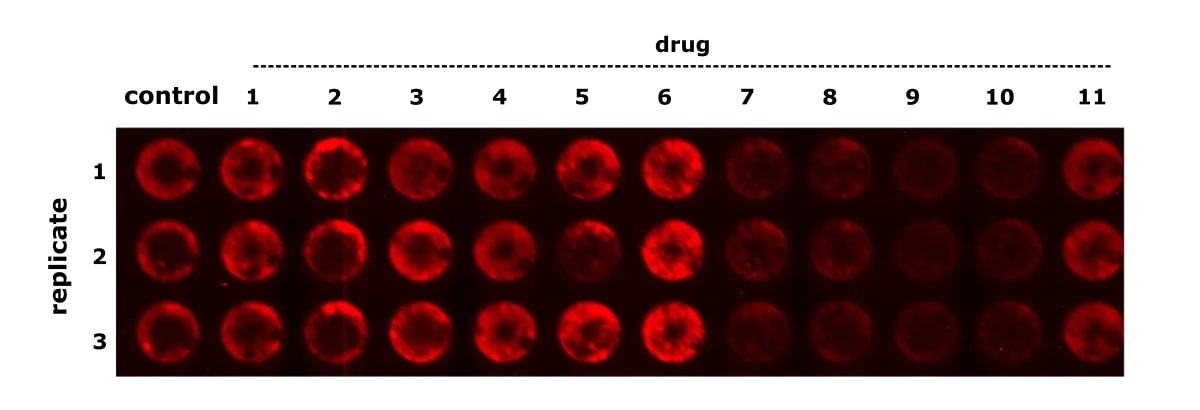
How Do In-Cell Westerns, Traditional Western Blots, and ELISAs Compare?
See Table 1 for a comparison of in-cell Westerns, traditional Western blots, and ELISAs. For more information on differences and similarities between Western blotting and In-cell Westerns, read this blog on the History of in-cell Westerns.
Table 1. Comparison between the properties of in-cell Westerns, traditional Western blots, and ELISAs.
Property | In-Cell Western | Traditional Western Blot | ELISA |
Target | Protein targets are inside fixed, whole cells. | Targets are purified from cell lysates or biological matrixes. | Targets are purified from cell lysates or biological matrixes. |
Detection | Targets are revealed using antibodies conjugated to near-infrared fluorophores. | Traditionally, Western blot targets are revealed via the antibody-conjugated enzymatic oxidation of luminol to chemiluminescent 3-aminophthalate. Fluorescence detection is also possible. | Targets are revealed using antibody-conjugated enzymes such as horseradish peroxidase or alkaline phosphatase that catalyze enzyme–substrate color change reactions. Chemiluminescence and fluorescence imaging are also possible. |
Throughput | High throughput as targets are analyzed in multi-well plates. | Throughput is limited by the capacity of the electrophoresis equipment and transfer apparatus. | High throughput as targets are analyzed in multi-well plates. |
Multiplexing | Simultaneous imaging of multiple targets is possible. | No option for multiplexing for chemical Western blots. Multiplexing is possible for fluorescent Western blots. | Simultaneous imaging of multiple targets is possible. |
Electrophoresis | No electrophoresis or transfer steps. | Electrophoresis and transfer provide target size information but can be hard to reproduce. | No electrophoresis or transfer steps. |
Visualization | A laser scanner is used to image targets. | Targets can be imaged using a laser-based scanner, CCD-based imager, or photographic film. | A plate reader capable of detecting the signal (e.g., chemiluminescence or fluorescence) is used. |
Advantages and Limitations of In-Cell Westerns
In-cell Westerns offer several distinct advantages over traditional Western blots. For example:
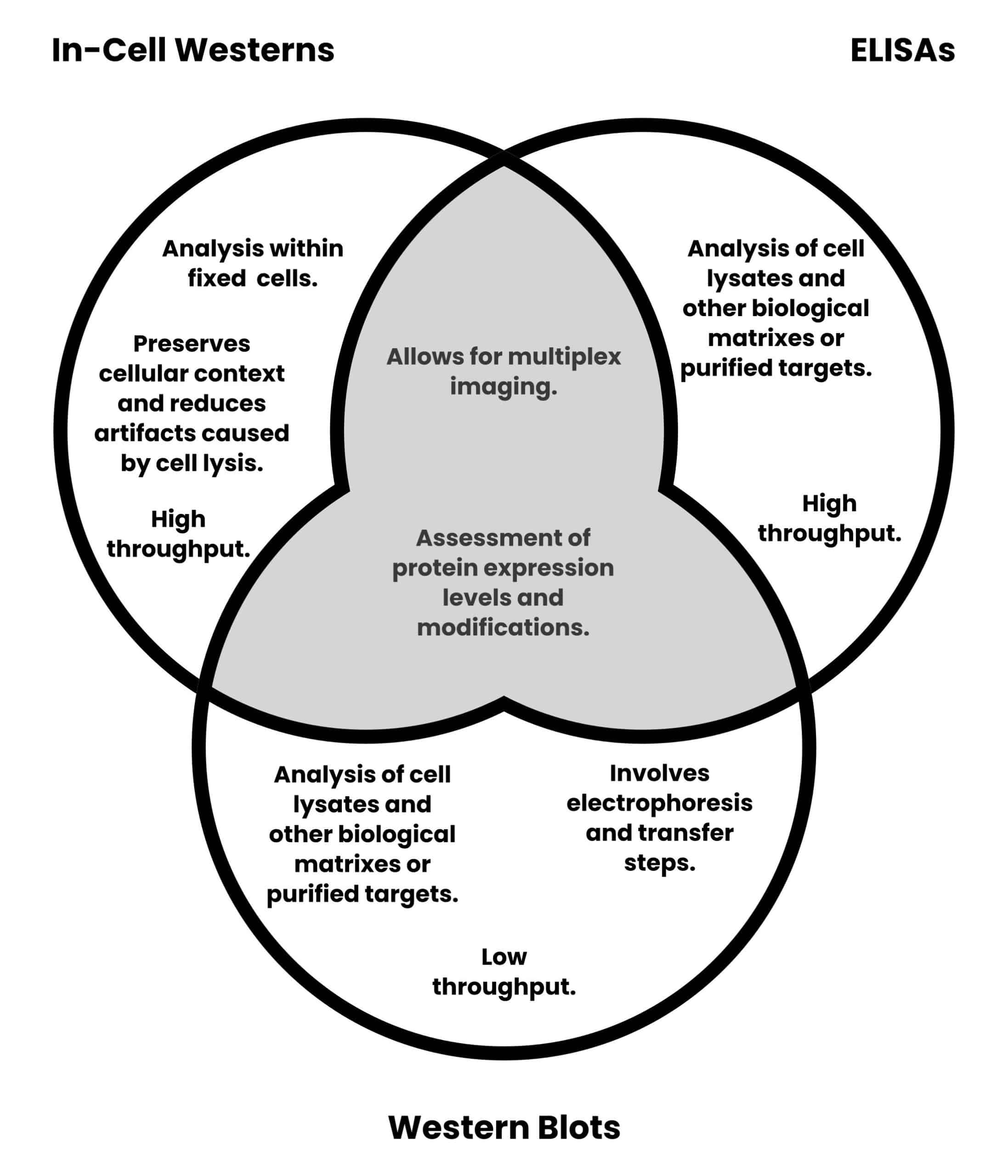
- Analysis within fixed cells preserves the cellular context and avoids artifacts caused by cell lysis.
- It offers higher throughput compared to traditional Western blotting.
However, there are certain limitations to consider when deciding if in-cell Westerns are the appropriate method:
- Not all antibodies can efficiently penetrate cells, potentially affecting the accuracy of results.
- Variability in cell fixation and permeabilization protocols may influence data consistency.
- The technique is not directly applicable to non-adherent cells, and extra protocol steps may be required.
Figure 2 provides a graphical comparison of in-cell Westerns, traditional Western blots, and ELISAs.
Tips for Successful In-Cell Westerns
Here are some crucial considerations to ensure the best results from in-cell Westerns.
Optimize Fixation and Permeabilization
Achieving optimal fixation and permeabilization is crucial for preserving intracellular structures and ensuring efficient antibody penetration. With that in mind, test different fixatives, concentrations, and fixation times to find the conditions that yield the best results for the specific cell type and target proteins used in each experiment.
Excessive fixation or permeabilization can lead to degraded antigens and increased background signal, while under-permeabilization can lead to reduced antigen accessibility.
Optimize Cell Seeding Density
Cell seeding is the initial step in preparing a culture for an experiment. It involves putting (seeding) the cells into a dish or substrate.
Optimizing the cell seeding density is critical because the density of cells at the seeding step influences subsequent cell growth and behavior. Care should be taken to ensure cells are not too crowded or sparse, both of which can affect protein expression levels.
Cell confluency is a measure of how much of the surface of a cell culture vessel is occupied by cells. It is often determined by eye from visual inspection with a microscope, although software is available that can determine confluency. A cell confluency of ~80% (meaning 80% of the vessel surface is occupied by cells) is often used for experiments, although this may differ depending on the objectives.
Optimize the number of cells needed for seeding to achieve the desired cell confluency. The number of cells required will differ between well sizes and cell lines, but properties such as how well the cells adhere to the multi-well plate and their doubling time must also be considered.
Validate the Antibodies
Ensure the antibodies used work for the intended application and have been validated for intracellular staining. Avoid antibodies exhibiting cross-reactivity and consider using pre-conjugated near-infrared fluorescent secondary antibodies where possible. This avoids having to conjugate antibodies to near-infrared probes manually.
Include Positive and Negative Controls
Positive and negative controls should be included in all experiments to validate specificity and sensitivity. Positive controls should contain cells expressing the protein of interest, while negative controls should include cells lacking the protein that otherwise share the same experimental conditions.
Normalize Data to Internal Controls and Total Cell Numbers
Accounting for variations in cell number is critical. Therefore, it is essential to normalize the data to internal controls, such as the total number of cells.
A nuclear stain such as DAPI can be used to normalize the signal from in-cell Westerns to the total number of cells in a given well. The Sapphire™ FL’s 375 Custom Optical Module enables imaging of common UV stains like DAPI.
For your next in-cell Western experiment, use a commercial kit to help you achieve brilliant in-cell Western results, like the new AzureCyto In-cell Western Kit from Azure. This kit is a complete system containing all the reagents necessary for staining fixed cells for total cell normalization and simultaneous detection of two biomarkers. It includes:
AzureCyto Permeabilization Solution: For removing membrane lipids and facilitating permeabilization of the nuclear membrane to allow the penetration of antibodies into cells for detecting intracellular and nuclear proteins.
AzureCyto Block Solution: Formulated for cell-based assays and fluorescence detection.
AzureCyto Total Cell Stain: A sensitive and linear stain that can be co-incubated with primary antibodies to streamline the detection process and reduce the number of assay steps.
AzureSpectra Secondary Antibodies: For simultaneous near-infrared detection of two biomarkers.
Imaging In-Cell Westerns Using a Laser Scanner
Laser scanners are the ideal choice for imaging in-cell Westerns, as they quantitatively measure the immunofluorescence from the bottom of multi-well plates.
The Azure Biosystems Sapphire™ FL Biomolecular Scanner is one of the highest resolution (down to 5 microns) and most versatile solutions available. (Figure 3)

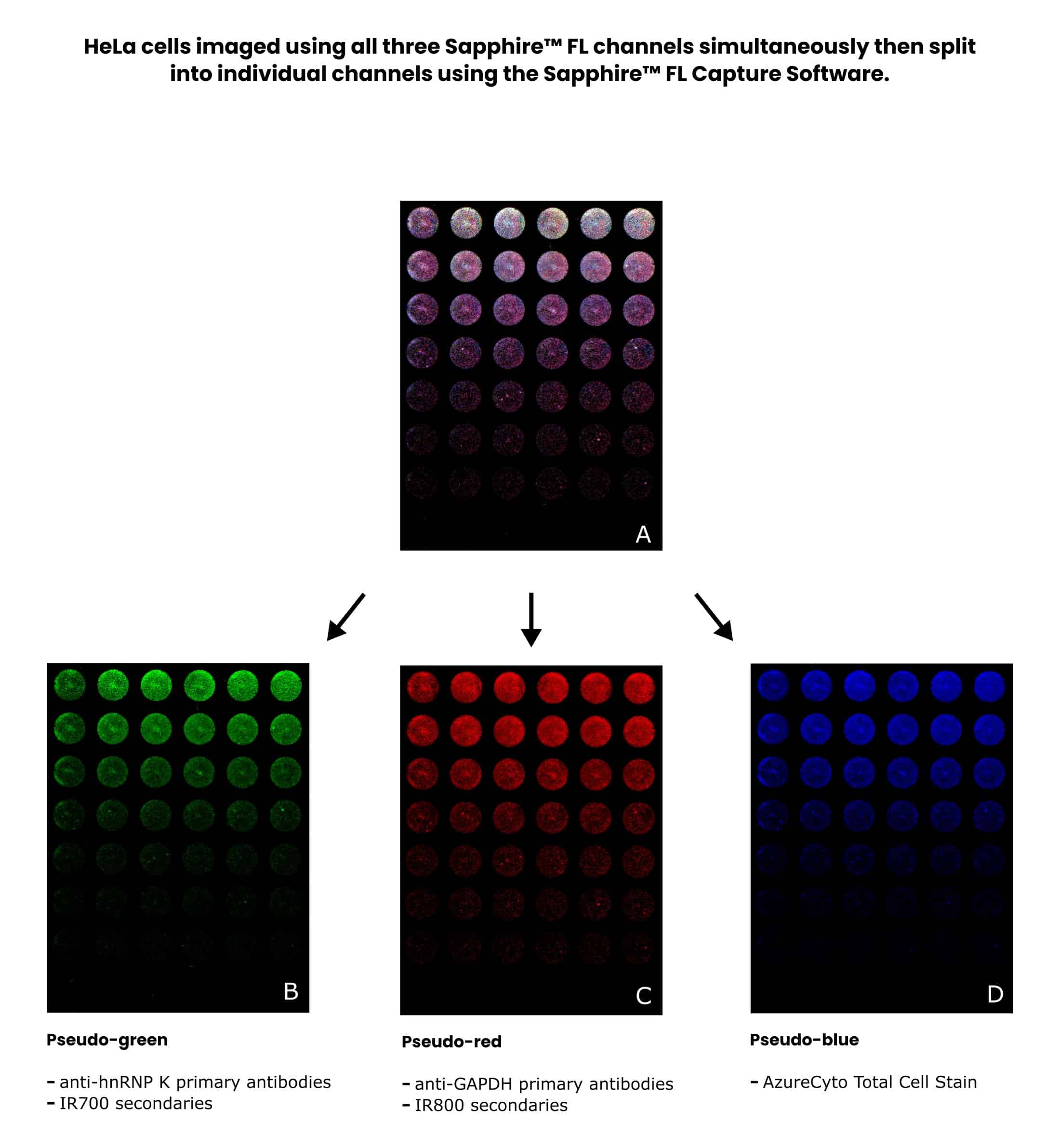
It has interchangeable and customizable laser and filter modules and supports the visualization of up to three targets and total cell normalization on the same sample.
The Sapphire™ FL software features an automated Z-scanning function to find the focal plane of cells with the click of a button. It can also generate stunning composite images of multiplex data and controls.
Figure 4 shows results for a multiplexed in-cell Western of HeLa cells imaged using a Sapphire™ FL Biomolecular Scanner.
In-Cell Westerns Summarized
By combining the high throughput nature of an ELISA with the specificity of a Western blot, in-cell Westerns can provide a vast amount of physiologically relevant data and are helpful in major branches of life science research such as drug discovery and pathophysiology.
For more information on in-cell Westerns or questions about the products available, visit the Azure Biosystems website.
References
- Boveia V and Schutz-Geschwender A (2015) Quantitative Analysis of Signal Transduction with In-Cell Western Immunofluorescence Assays. In: Kurien B and Scofield R (eds) Detection of Blotted Proteins. Methods Mol Biol 1314. Humana Press, New York
- Lu LD and Salvino JM (2023) The In-Cell Western immunofluorescence assay to monitor PROTAC mediated protein degradation. In: Burslem GL (ed) Targeted Protein Degradation. Meth Enzymol 681. Academic Press, Cambridge, MA
- Hoffman GR et al. (2010) A high-throughput, cell-based screening method for siRNA and small molecule inhibitors of mTORC1 signaling using the In Cell Western technique. Assay Drug Dev Technol 8(2):186–99
- Disch JS et al. (2021) Bispecific Estrogen Receptor α Degraders Incorporating Novel Binders Identified Using DNA-Encoded Chemical Library Screening. J Med Chem 64(8):5049–66
- Wan Y, Zhou Z, Yang Y, Wang J, and Hung T (2010) Application of an In-Cell Western assay for measurement of influenza A virus replication. J Virol Methods 169(2):359–64
- Egorina EM, Sovershaev MA, and Østerud B (2006) In-cell Western assay: a new approach to visualize tissue factor in human monocytes. J Thromb Haemost 4(3):614–20
- Filardo S, Di Pietro M, Pasqualetti P, Manera M, Diaco F, and Sessa R (2021) In-cell western assay as a high-throughput approach for Chlamydia trachomatis quantification and susceptibility testing to antimicrobials. PLoS One 16(5):e0251075







