In a previous article, we went over the basic understanding of the inner workings of a flow cytometer. It’s important to grasp the types of measurements that are being made and, perhaps more importantly, what measurements are NOT being made. For simplicity’s sake, we’re going to frame this discussion in terms of a classical flow cytometer; think of a BD FACScan or FACSCalibur. Of course, there are very recent advances in how flow cytometrists are thinking about scatter and fluorescence and we’ll briefly touch on those at the end, but for now, let’s keep it simple.
A Brief Recap of Flow Cytometry Basics
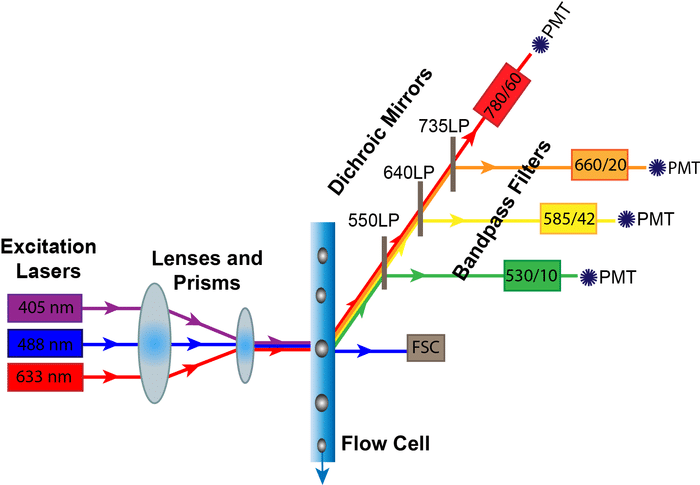
As a cell passes through the interrogation point of a flow cytometer it both scatters light and, any fluorophores on or in the cell, will absorb the incident light and emit photons in a range of wavelengths. These photons terminate at a detector and a current is generated in the detector proportional to the amount of incident light. This process is repeated for each cell and for every laser employed on the instrument.
What is Scatter?
An object passing through any light source will scatter light. This same process, which allows us to observe certain properties of objects in the real world, also allows us to measure properties of cells as they pass through a cytometer. We’re simply replacing “object” with “cell,” our “eye” with a “photon detector” and our “brain” with an “electronics system and computer”.
Forward Light Scatter
A laser is focused on a point on the flow stream where cells will eventually pass through. When no cell is in the path of the laser beam, the beam passes through the stream uninterrupted and is blocked from swamping the forward scatter detector by a thin obscuration bar resulting in no signal reaching the detector. When a cell passes through the laser, light is refracted in all directions, but the light that is refracted in the forward direction, that is along the same axis that the laser is traveling, will miss the obscuration bar and reach the forward scatter detector.
In general, it can be observed that large objects will refract more light than smaller objects leading to high forward scatter signals (and vice versa).
Light scatter is also dependent on a change in refractive index between the sheath fluid, the sample buffer, and the cell itself. Large disparities in the indices of refraction between these different planes could lead to inaccuracies in relative cell sizing. For example, a sample sitting in an ethanol fixative will scatter light very differently than the same sample in PBS. This can partly be explained by the difference in refractive indices of H2O and ethanol but is also due to changes in the cell membrane post fixation. So although we tend to use the shorthand, forward scatter is size, care should be taken not to take this too literally.
Side Light Scatter
Scattered laser light is also detected orthogonal to the incident laser beam. This side-scattered light is proportional to the overall size of the cell, but is also affected by things like the internal complexity of a cell or the smoothness of a cell’s membrane. It can be inferred that a rough cell (e.g. a cell undergoing apoptosis) or a cell with great internal complexity (e.g. an eosinophil with many granuoles) would have high side scatter signals. Again, light scattering is much more complicated than what’s presented here, but we tend to shorthand side scatter as internal complexity.
Hopefully, you can plainly see the immense power of even a simple correlation of these two properties, forward and side laser light scatter. It’s no wonder most flow cytometry practitioners make this their very first plot when analyzing data.
It’s all Relative: The Relativity of Fluorescence Measurements
Before we move on to fluorescence, it’s important to understand one crucial point to flow cytometry measurements; they’re all relative. The dots that show up on a plot, or the hills present on a histogram have no absolute value. They can easily be moved up and down the scale according to the voltage applied to a detector. Therefore, any measurement in flow cytometry must be related to some baseline or control. We can only determine if the dots shown on our light scatter plot represent a large cell if we compare it to some other cell or reference material (taking into consideration the caveats presented above).
Likewise, the fluorescence signals observed can only be understood in light of a negative or unstained control sample. Proclaiming that your stained sample has an average fluorescence intensity of 1000 units means absolutely nothing until you compare it to an unstained control.
Fluorescence Measurements
The fluorophores present on or in the cell as it passes through the laser beam add to the cumulative signal attributed to that cell. In other words, the fluorescence signal observed for a given cell is a measurement of the total fluorescence of the cell regardless of the localization of the fluorophore. This, evidently, is the downside of conventional flow cytometers. While it’s very easy to tell if there is fluorescence emitting from the cell you cannot tell from where the fluorescence is coming; that is, the fluorescence localization.
Image Cytometry
Fear not cytometry enthusiasts, for where conventional flow cytometry has been found wanting, image cytometry has filled in the gaps to provide both the quantitative power of flow cytometry and the fluorescence localization powers of imaging in a single platform – a topic we’ll be sure to cover in the near future. Although the default use of a flow cytometer relies on a relative measurement compared to a control, it is possible to calibrate the fluorescence scale into a more quantitative metric.
Absolute Quantification of Flow Cytometry Measurements
The world of quantitative flow cytometry (QFCM) is a complicated one, but there are tools that make this possible. Probably one of the best-known calibrators is the MESF standard. MESF beads convert the relative fluorescence scale into a scale of Molecules of Equivalent Soluble Fluorophore. This basically means that your fluorescence scale reads out fluorescence in the FITC channel as a signal equivalent to 1000 molecules of fluorescein. One can then calculate the number of molecules of FITC on each antibody using spectrophotometry and therefore the number of antibodies bound per cell. Now, one is able to report fluorescence data in an absolute manner instead of a relative one.
The Future of Flow Cytometric Measurements
In recent years greater attention has been given to the complexities involved in light scattering, employing both the Mie solution to Maxwell’s equation as well as Rayleigh’s scattering principles to push the limit of detecting submicron particles. Read Evolution Flow: The Historical Background of Flow Cytometry to discover more about Rayleigh’s scattering principles. In addition, polarized light has been given greater notice as well as multiple angles of scatter collection.
Another area that has experienced resurgence is electrical impedance measurements first described by Wallace Coulter. As we’ve discussed previously, the Coulter Principle allows for very accurate size measurements and particle counts from a suspension. Although the principles employed are very old and well established, the implementation in consumer hardware remains elusive. As these complexities begin to permeate the field, we’ll hopefully reap the benefits of such advances.
Originally published on April 1, 2013. Updated and Revised on July 29, 2015.