Ever wonder what those small colonies, like satellites, surrounding a larger E. coli colony on your LB with ampicillin plates were? Or why, when you picked that colony, it never had the plasmid you just transformed? Well, it’s because those satellite colonies are “protected” from the ampicillin by the big colony. Read on for more…
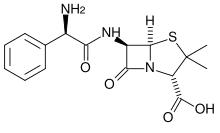
Ampicillin is commonly used as a selection marker for plasmids in gene cloning and protein expression in E.coli and other bacteria. While it serves its purpose, there can be problems using this selection marker if the user is unaware of its limitations. This article provides a quick overview of what those limitations are and how to avoid them.
The basis of ampicillin selection is that the plasmid-borne bla gene, that encodes beta-lactamase, hydrolyzes and inactivates of the antibiotic. Here’s the problem: beta-lactamase is secreted by the bacteria. The resulting build-up of extracellular beta-lactamase can inactivate the ampicillin in the culture medium, removing the selective pressure and allowing satellite colonies to grow, if the culture is not handled properly.
In liquid cultures, this means that a portion (possibly a very large portion) of the cells no longer have the plasmid, giving poor yielding plasmid preps, protein expression etc. On agar plates, ampicillin degradation can lead to the formation of satellite colonies on transformation plates. Satellite colonies have not taken up the the bla-containing plasmid. The satellites form because the beta-lactamase released by the bla-expressing larger colony degrades the ampicillin in the vicinity of the colony. The satellites are not necessarily a problem as they will not grow when transferred to a medium containing fresh ampicillin.
Enjoying this article? Get hard-won lab wisdom like this delivered to your inbox 3x a week.

Join over 65,000 fellow researchers saving time, reducing stress, and seeing their experiments succeed. Unsubscribe anytime.
Next issue goes out tomorrow; don’t miss it.
So how do you avoid plasmid loss when using ampicillin as a selection marker? Here’s how:
- Don’t allow liquid cultures to saturate for too long. I would recommend never growing cultures higher than OD600=3 (in LB)
- If you are using a starter culture, always pellet and re-suspend the starter culture in fresh, antibiotic-free medium before innoculating the main culture. This is to remove the secreted beta-lactamase from the medium.
- Use a higher ampicillin concentration if you are experiencing problems. I would recommend using 200 micrograms per mL or higher. This makes it harder for the beta-lactamase to inactivate all of the ampicillin and is especially useful for avoiding satellite formation.
- For the same reason, never use old ampicillin stocks or plates as the ampicillin will have broken down somewhat, giving a reduced effective ampicillin concentration.
- If all else fails, switch to carbenicillin selection. This antibiotic is also inactivated by beta-lactamase but more slowly than ampicillin is. For this reason carbenicillin selection is far more effective than ampicillin. Unfortunately it is much more expensive!
Do you have any tips on working with ampicillin? If so, please leave a comment.
Originally published on May 9, 2011. Revised and updated on July 29, 2015
You made it to the end—nice work! If you’re the kind of scientist who likes figuring things out without wasting half a day on trial and error, you’ll love our newsletter. Get 3 quick reads a week, packed with hard-won lab wisdom. Join FREE here.