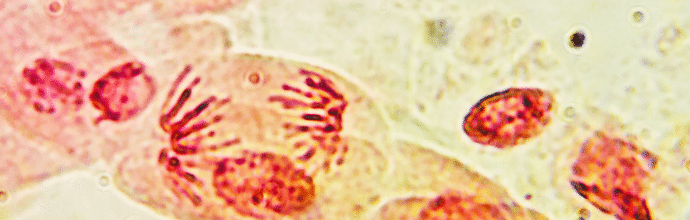
The last two decades have seen a dramatic increase in the number of publications using immunohistochemistry (IHC) as a research tool to identify the spatial location of proteins of interest within cells, tissue sections and whole-mount preparations.
Grinding and binding
The advantages over ‘grind and bind’ methods are apparent, but the very best results will only be obtained if the technique is treated as part of a process and not an event in itself. I don’t know of anyone who does immunohistology as a stand-alone technique. It is part of a process involving tissue fixation, processing and sectioning. Immunohistology is not the end game, microscopic imaging is. Only by considering all the stages in the process will well-stained cells or sections be obtained which will ultimately lead to the best images being acquired.
The two starting techniques
There are many techniques and reagent technologies available to today’s researcher. When I first started doing IHC within a diagnostic laboratory, we used two basic techniques. The PAP method (peroxidise anti peroxidase) and the indirect method;
- The PAP method was the most sensitive as it was able to have more Horse Radish Peroxidase (HRP) enzyme conjugated to it resulting in a stronger reaction when incubated with its substrate, Diaminobenzidene (DAB). I still see researchers using the indirect method on a regular basis (for Western Blots and IHC) as it is an easy technique to perform. Basically, a primary antibody is incubated against the target protein and then a secondary antibody with a label of choice (enzyme, fluorochrome, nanogold etc.) binds to the primary antibody which can then be visualized.
- The indirect method has great versatility in that you can visualize your target protein by merely changing the labelled secondary, which is much more versatile than having primary antibodies directly labelled. The PAP method soon fell out of favour as avidin-biotin detection systems and streptavidin detection systems were developed. These proved to be more sensitive and quicker to perform.
The avidin-biotin or streptavidin systems also provide many different streptavidin labels. The only real downside is that certain tissues contain high levels of endogenous biotin within them which, of course, your avidin or streptavidin will bind to with high affinity giving false positive staining. Avidin-biotin blocking reagents can be applied to cells or sections eliminating this staining, but increasing both time and expense.
Constant evolution
Immunodetection is a field which is constantly evolving and researchers will have their own personal preferences.
Currently I use two of the most sensitive detection systems available today;
- The first is polymer based reagents where a secondary antibody is bound to a polymer backbone to which a large amount of enzyme can be conjugated- usually HRP or alkaline phosphatase. This is particularly useful for colormetric colocalization studies. Several manufacturers produce them and are available for mouse, rabbit, rat, goat and sheep primaries (but also others I suspect). Polymer systems are heavily used on robotic staining systems and don’t have the pitfalls associated with streptavidin/avidin based systems.
- My second system of choice is tyramide based detections. Tyramide based detections require an HRP intermediate. I detect the primary antibody of interest using either an HRP conjugated polymer reagent or conventional secondary. When tyramide is applied to this, the HRP catalyses the deposition of labelled tyramide which, in turn, gives a large degree of signal amplification around the site of antibody binding and your protein of interest. Again, this has great sensitivity and because of the availability of tyramides, there is an extensive range of multi-colored or fluorescent end points.
Tyramide systems also have a unique advantage in colocalization studies, but that’s another article…
Exciting times ahead!
The field of molecular tissue probing is entering a really exciting time and I can only envisage its popularity increasing over the next few decades. Techniques such as immunohistochemistry, immunofluorescence, immunohistochemical and immunofluorescent colocalization, in-situ proximity ligation, in-situ hybridisation and RNAscope give today’s researcher unprecedented flexibility for examining the spatial location of protein, mRNA and DNA within cells and tissue. Furthermore, with the emergence of super resolution microscopy we will have the ability to examine minute structures in unprecedented detail.
One stage in a long process
If you take nothing else from this article please remember that molecular tissue probing is still only a stage in the process and all the stages must be considered with joined up thinking to produce the ultimate results!