In biopharmaceutical R&D, sensitivity is everything. It’s what enables you to detect low analyte concentrations, resolve subtle binding events, and accurately measure kinetic and affinity parameters. These insights lay the groundwork for confident decision-making at every stage.
For years, surface plasmon resonance (SPR) has set the standard for sensitivity in label-free assays. But SPR’s fluidics-based design adds complexity to workflows. Biolayer interferometry (BLI), by contrast, delivers speed and simplicity—though traditionally with a trade-off in sensitivity.
That balance is about to change, making it finally possible to detect low-affinity protein binding without SPR.
A Bitesize Primer: SPR vs. BLI
Both surface plasmon resonance (SPR) and biolayer interferometry (BLI) are label-free technologies used to study biomolecular interactions, but they work in very different ways.
- SPR relies on microfluidics and a gold-coated sensor surface. When molecules bind, changes in the refractive index are detected as shifts in a plasmon resonance signal. This design gives SPR its hallmark high sensitivity, but also makes systems more complex, maintenance-intensive, and costly to operate.
- BLI, by contrast, uses dip-and-read fiber optic biosensors. The binding of molecules at the biosensor tip causes an interference pattern shift in reflected light, which is tracked in real time. The absence of fluidics makes BLI systems faster, simpler, and lower maintenance, with the added advantage of handling many samples in parallel.
The trade-off has always been sensitivity: SPR could see what BLI sometimes missed. But that gap is now narrowing with advances like the new Octet® R8e, which brings near-SPR sensitivity into the streamlined BLI workflow. This makes it possible to detect low-affinity protein binding without SPR.
Biolayer Interferometry (BLI) Reaches New Levels of Sensitivity
Recently, the Octet® BLI instrument family welcomed a new addition, which combines near-SPR sensitivity with the flexible, high-throughput workflow that BLI users already rely on.
It’s called the R8e (Figure 1) and is an enhanced version of its widely used eight-channel Octet® R8. But what makes this launch particularly exciting is that it unlocks BLI’s full potential for early-stage biologics screening, affinity ranking, and biosimilar characterization. With its improved sensitivity you can use less material and measure small differences.
How? The system’s significantly improved signal-to-noise ratio lowers both the limit of detection (LOD) and the limit of quantitation (LOQ), while also expanding dynamic range. This means you can detect low-affinity protein binding without SPR.
Figure 1. The R8e delivers dramatically improved sensitivity over traditional BLI, enabling detection of human IgG at concentrations as low as 3.9 ng/mL. Faster signal resolution means you can measure weak interactions in seconds instead of minutes.
Let’s look at the data.
Detect More with Less Sample
Detecting low analyte concentrations is a critical challenge in drug discovery and diagnostics. Greater sensitivity means you can work with smaller sample volumes and still measure weak interactions or low-abundance targets.
As shown in Figure 2, the R8e can quantify concentrations as low as 3.9 ng/mL (26 pM) and detect signals above the LOD in just 25 seconds. This means you can get faster, clearer answers, enabling earlier and more confident decision-making.
Practical Application: Detecting IgG at just 3.9 ng/mL means you can work with precious patient-derived samples or hard-to-express proteins without scaling up production. Early detection lets you make go/no-go decisions sooner, saving time and resources.
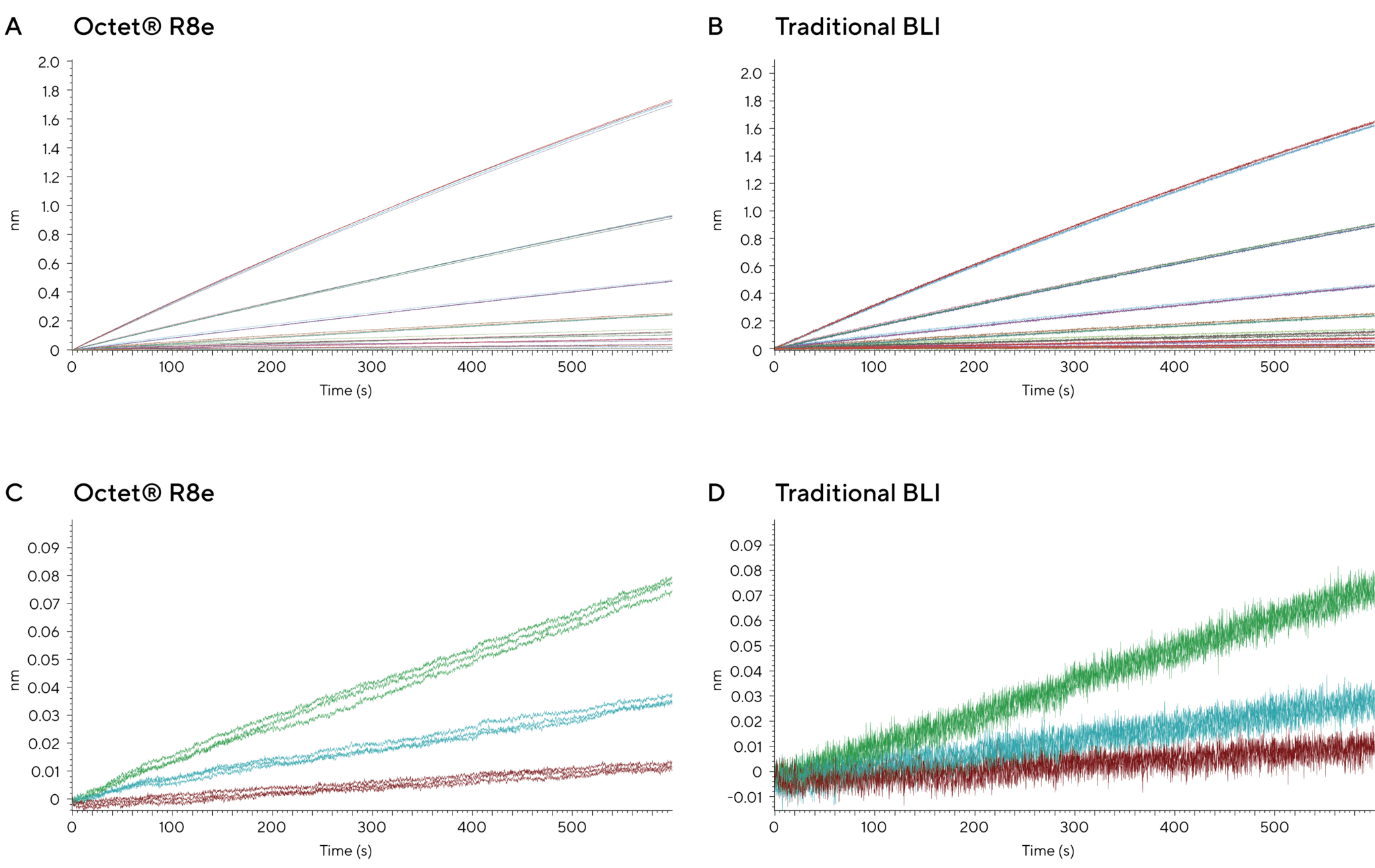
Figure 2: (A) Reference-subtracted data showing binding of human IgG (3.9 – 500 ng/mL) to Octet® ProA biosensors on the Octet® R8e (A) and traditional BLI (B). The improved signal to noise of the Octet® R8e is highlighted when comparing the lowest concentrations of hIgG (3.9, 7.8 and 15.6 ng/mL). (C) Octet® R8e displays decreased noise levels and corresponding separation of data points which allows accurate determination of analyte concentrations in a shorter period of time compared to the traditional BLI (D).
Measure Small Molecules Without Switching Platforms
Small molecule assays traditionally used SPR due to BLI’s sensitivity limits. Not anymore.
Figures 3 and 4 demonstrate clear, well-resolved sensorgrams for furosemide (330.7 Da), sulpiride (341.4 Da), and benzenesulfonamide (157.2 Da). Even the fine kinetic details during the early stages of association and dissociation are visible.
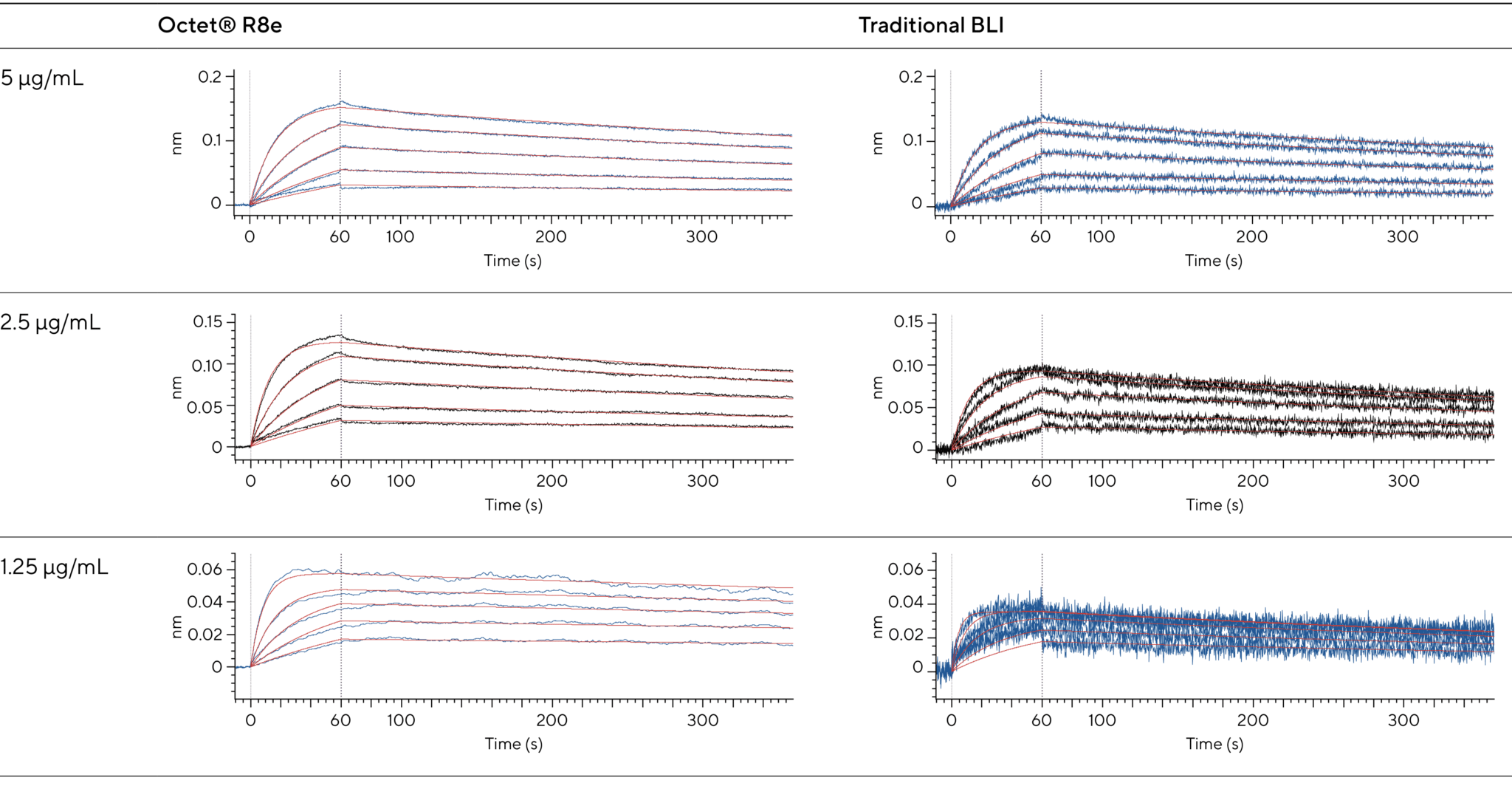
Figure 3: Even at responses below 0.1 nm, the R8e provides distinct, noise-free signals, revealing analyte concentration differences that were previously undetectable on traditional BLI systems. Here, insulin peptide (5,808 Da) binding to a capture antibody shows distinct responses for each analyte concentration below 0.06 nm (1.25 μg/mL ligand concentration) when assessed on the Octet® R8e. For all figures, Savitzky-Golay filtering is not used, highlighting the improvement in signal-to-noise on the Octet® R8e system.
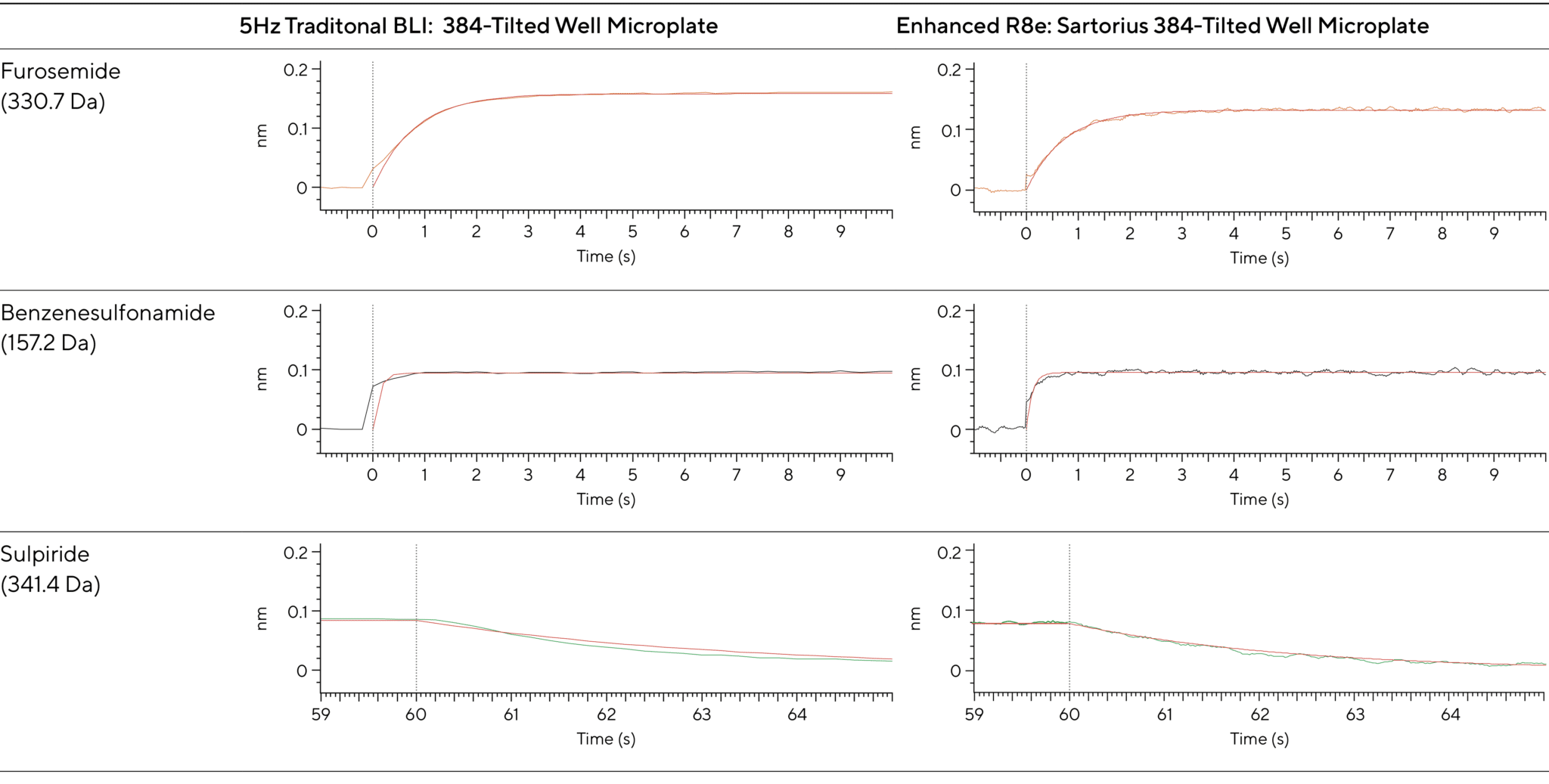
Figure 4: Enhanced data capture during binding and dissociation reveals molecular interactions once hidden from view, providing deeper insights into kinetics and affinity. It improves accuracy in determining association and dissociation rate constants, offering deeper insights into molecular interactions. The Octet® BLI R8e system enhances sensitivity and flexibility, advancing drug discovery by revealing unseen interactions.
Conserve Samples and Cut Costs Without Sacrificing Throughput
Sample conservation is essential when working with hard-to-express proteins or rare patient-derived materials. At the same time, throughput demands are increasing
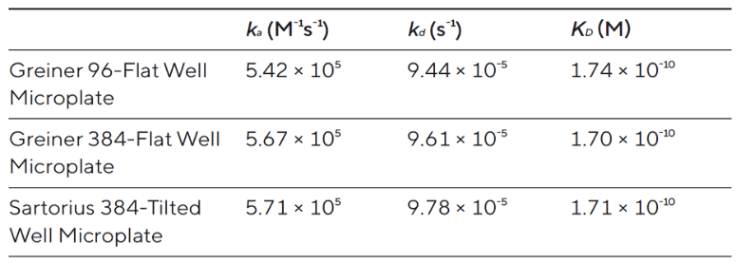
The R8e supports 384-well plates and needs just 40 μL per well, helping you cut down on sample use without sacrificing data quality. Table 1 shows consistent kinetic and affinity values across multiple plate formats, confirming reliable performance.
Practical Application: By using only 40 µL per well in 384-well plates, you can stretch rare or costly samples across more assays. This is especially valuable when working with limited clinical material or expensive recombinant proteins.
Table 1: Comparable kinetic and affinity results across multiple plate formats demonstrate the R8e’s reliability while reducing per-sample cost.
Run Long Assays Without Evaporation
Extended kinetic runs are essential for capturing slow off-rates and full dissociation events, but evaporation often undermines data quality.
The R8e tackles this with a new evaporation control system designed specifically for 96-well plates. Figure 5 shows how the system preserves sample integrity for up to 16 hours, even during extended association or dissociation phases. This is especially valuable for antibody maturation studies or late-stage characterization where precise affinity data is critical.
Practical Application: The ability to run reliable 16-hour assays opens the door to capturing very slow off-rates. This makes the R8e ideal for antibody maturation studies, where precise affinity measurements guide therapeutic candidate selection.
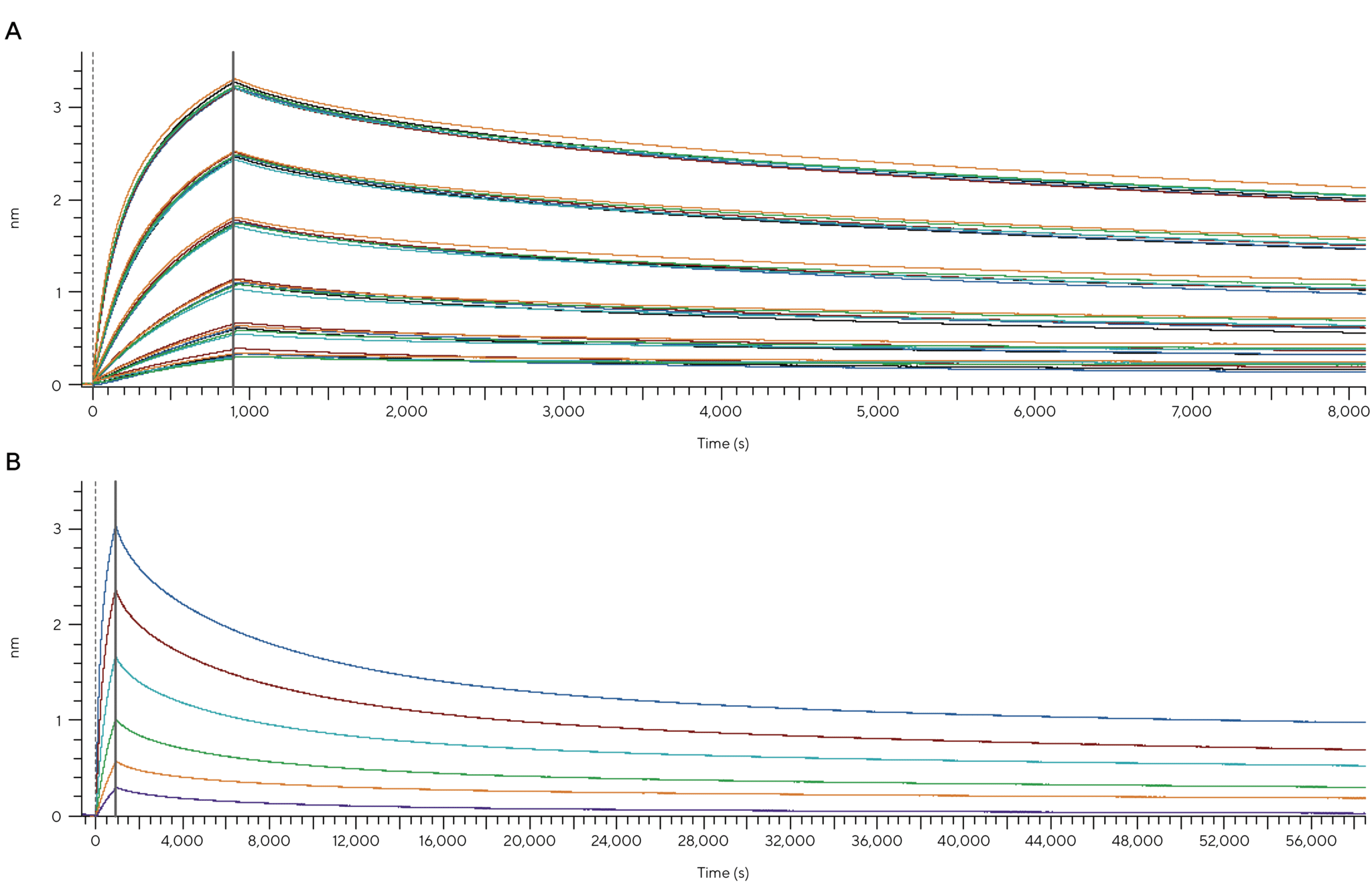
Figure 5: With advanced evaporation control, the R8e maintains sample stability for 16-hour assays, delivering precise off-rate measurements for antibody maturation and late-stage characterization. (A) Detection of Human IgG Goat Fab using FAB2G Biosensors on the Octet® R8e system. Six replicates with assay parameters of 1000 rpm speed, 900 s association time and 7,200 s dissociation minutes per sample were performed at 30 °C. Across the 16-hour assay, association and dissociation rate constants were determined with percentage CVs below 7.7% for all replicates. (B) Single replicate of Human IgG Goat Fab detection using FAB2G Biosensors with an extended dissociation time of 16 hours (57,600 s) allows accurate determination of dissociation rate constants due to minimized evaporation.
Enjoy Vetted Enterprise-scale Workflow Efficiency
Whether you’re running a single assay or managing a regulated R&D pipeline, efficiency and reproducibility are non-negotiable.
Like all Octet® systems, the R8e eliminates common disruptions like clogs and high-maintenance fluidics. It simplifies assay setup and sample recovery, while supporting 21 CFR Part 11 compliance for regulated environments. The shared biosensor catalog also makes it easy to scale across labs and teams.
Practical Application: Because the system avoids fluidics, you’ll spend less time troubleshooting clogs or maintenance issues. In regulated labs, 21 CFR Part 11 support means you can expand assays into validation workflows without changing platforms.

Dip-and-read biosensors accelerate workflows while ensuring consistency across sites, programs, and regulated pipelines
So, Is SPR Still Necessary?
With the Octet® R8e, you gain near-SPR sensitivity without the burden of complex fluidics. From early discovery to regulated workflows, the system extends BLI’s reach into new territory, giving you deeper insights, faster turnaround, and lower costs.
So the question remains: if you can detect low-affinity protein binding without SPR—do you really need SPR?
For R&D teams under pressure to move quickly, conserve resources, and deliver better data, the R8e offers a clear path forward.
Discover more at www.sartorius.com/octet-r8e