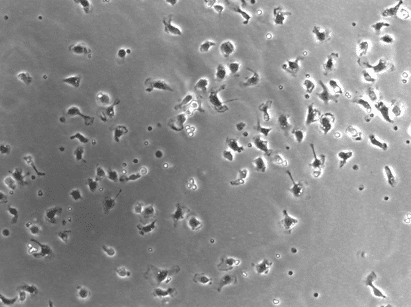
When it comes to culturing primary cells, every cell type is different and getting familiar with the ins and outs of the cells can take a long time. I have worked extensively with primary Schwann cells during my PhD and want to cover some of the basics of primary Schwann cell culture in this article.
Choosing Your Schwann Cell
There is a wide range of different protocols out there detailing how to obtain primary Schwann cells. But before you begin, take a moment to think about your experiment and what exactly you want to achieve. It is very important to choose the correct type of primary Schwann cell for your specific question, as they come with different advantages and drawbacks. I have personally worked with what are probably the three most commonly used types of primary Schwann cells: rat Schwann cells derived from neonatal sciatic nerves, mouse Schwann cells derived from embryonic dorsal root ganglia, and mouse Schwann cells derived from early postnatal sciatic nerves.
So, let’s have a look at the pros and cons of these three types of primary Schwann cells, so you can decide which one is the right type for your needs.
- Rat Schwann cells from neonatal nerves (rat SCs):
Pros:
Rat SCs are highly proliferative: you can expand them for about 10 – 12 passages in the right culture conditions, freeze and thaw them, and transfect and infect them with viruses. You can also differentiate them in vitro. Last, but certainly not least, they retain the capacity to myelinate axons when co-cultured with neurons.
Cons:
You rarely find transgenic rats, so in most cases you have to do genetic modifications with siRNAs or a similar method for these cells. Also, there may be inherent differences between Schwann cells of mice and rat (or human for that matter), so keep that in mind if you do the rest of your project with a different model.
Recommended use:
Studying signaling pathways, assessing the effect of drugs on myelination, protein interaction studies. - Mouse Schwann cells from embryonic dorsal root ganglia (embryonic mouse SCs):
Pros:
As long as you don’t have a major impairment in the proliferation of Schwann cells, you can derive these cells directly from your genetically modified mice. Embryonic mouse SCs are proliferative, and can be expanded for about 3 – 4 passages. You can freeze and thaw them, and it is possible to transfect them or infect them with a virus, although both at low efficiency. You can also differentiate them in vitro.
Cons:
If you want to derive them from your genetically modified mice, be aware that mouse SCs are isolated quite early in development (typically embryonic day 13.5), and not all Cre lines have recombined at this point. We have had good success with desert hedgehog-Cre but only moderate recombination efficiency in the cells when using myelin protein zero-Cre. Unfortunately, I have yet to find a protocol that enables these cells to form myelin in co-cultures with neurons.
Recommended use:
Protein analysis in genetically modified Schwann cells, assessing the effects of drugs on viability, proliferation, or protein/mRNA expression. - Mouse Schwann cells from early postnatal sciatic nerves (postnatal mouse SCs):
Pros:
You can derive these cells directly from your genetically modified mice. When examining all the primary Schwann cells, these are probably the closest to the in vivo environment. You can infect these cells with virus, although at low efficiency.
Cons:
The proliferation rate is low, so expanding and passaging is not possible. Therefore, they are usually only used as acute short-term cultures. Due to the low proliferation of the Schwann cells, fibroblasts in the culture can be a real problem if you want to keep them for longer than 24h. Fibroblasts are always a problem in primary Schwann cell cultures because: a) they proliferate more rapidly than Schwann cells; b) Schwann cells don’t grow on top of fibroblasts, so they take away valuable space in your culture dish; and c) they will “contaminate” DNA or protein extracts from your cultures, which you would like to be as pure as possible.
Recommended use:
Studying the effect of a genetic modification on Schwann cell properties such as process extension, proliferation, and survival.
Conclusion
Each type of primary Schwann cell culture has some unique properties and is useful in some experimental settings. I hope this overview helped you get an idea of the strengths and weaknesses of each type of culture and helps you in deciding how best to design your experiment(s) for optimal results. For more information on growing Schwann cells, read our top 5 tips for Schwann cell culture.
Further Reading
Example protocols for primary Schwann cell preparation:
Kim, H.A., and P. Maurel. 2010. Primary Schwann Cell Cultures. In Protocols for Neural Cell Culture: Fourth Edition. L.C. Doering, editor. Humana Press, Totowa, NJ. 253-268.