What Are Quantum Dots?
Quantum dots were discovered in the early 1980s. However, it was not until the late 1990s that their use in biological applications was suggested.1
Quantum dots are semiconducting nanocrystals made of artificial atom clusters. Their size generally ranges from 2 to 20 nm. Size is crucial for their physical properties because it sets the boundaries for electron motion within them.
The essential structure of a quantum dot consists of a core and a shell. The core usually contains cadmium mixed with selenium or tellurium, whilst the shell is made of zinc sulfide. The properties of the core-shell interface greatly impact the quality of the optical signal.2 There may be further coatings on the outside of the shell depending on the particular application.
How Do They Work?
In essence, quantum dots are fluorophores—they absorb and emit photons of light at distinct wavelengths. However, unlike many other fluorophores, quantum dots do not depend on electron transitions.
Enjoying this article? Get hard-won lab wisdom like this delivered to your inbox 3x a week.

Join over 65,000 fellow researchers saving time, reducing stress, and seeing their experiments succeed. Unsubscribe anytime.
Next issue goes out tomorrow; don’t miss it.
Let’s briefly recap on traditional fluorophores. Upon being hit by a light source, such as a laser, electrons jump from their resting state to a maximal energy level. Since this is not sustainable, electrons readily fall to a more stable energy level, the fluorophore undergoes a conformational change, and energy is released as heat. When the electrons reach their resting state, the remaining energy is released as fluorescence (i.e., light emission at a higher wavelength than the excitation source).
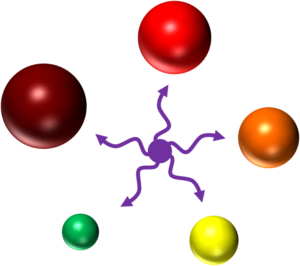
Quantum dots on the other hand create excitons within their core and shell analogous to the excited state of a fluorophore.3 Their size is proportional to the energy of their excitons—the larger the quantum dot, the longer the wavelength of the emitted fluorescence (see image 1).4
What Can the Quantum Dots Be Used For?
There are multiple uses for quantum dots. Outside of the lab you may find them in photovoltaics or electronic displays, in the lab they can be used for fluorescence-activated cell sorting (FACS) or fluorescence microscopy.
Quantum Dots in FACS
A flow cytometer is a piece of equipment which analyzes and sorts single cells based on predefined criteria e.g. size, granulation or expression of specific cell surface protein markers. In a process called hydrodynamic focusing, a flow cytometer lines up cells from a suspension in single file using sheath fluid.
Aligned cells pass a light source, usually a laser, and the flow cytometer produces quantitative and qualitative data about the cells’ size, shape and fluorescent labelling. Some instruments sort cells into new test vials for further analysis. For many years, fluorophores have been the dominant tool in cell labeling for FACS. In the last decade, quantum dots have entered the arena.5
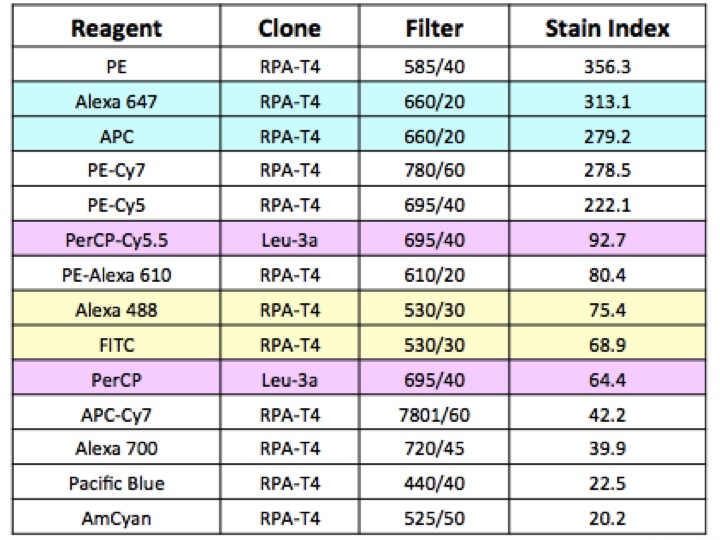
The absorbance spectra of quantum dots increases dramatically at shorter excitation wavelengths. They are optimally excited by a violet laser between 405–407 nm. Their narrow and symmetrical emission peaks do not change with different excitation sources.
Image 1: All quantum dots can be excited by the violet laser to emit fluorescence at different wavelengths dependent on their size. Credit: Dr. Christiane Wirrig
Similarly to fluorophores, quantum dots can be coupled to biomolecules such as antibodies, streptavidin or oligonucleotides to detect cell markers. As such, they represent an alternative labeling option in situations where traditional fluorophores underperform.
Advantages of Quantum Dots
Quantum dots have a lot going for them. Here is a brief overview of their main selling points:
- Possibility to multiplex: Since all quantum dots can be excited by just one laser, multicolor analysis is possible with simpler and potentially cheaper FACS machines. Designing multiplex set-ups with up to six different colors greatly maximizes the use of either the 405 nm (violet) or 488 nm (blue) laser.
- Photostability: An exciton’s lifetime can last up to microseconds, whilst a traditional fluorophore signal typically lasts only 1-10 nanoseconds. This difference is immense and allows long-term imaging experiments that would otherwise be restricted by signal loss through photobleaching. In addition, you can fix samples, which is very advantageous in follow-up analyses after in vivo studies. Fluorescence stability also means that quantum dot reagents potentially have longer shelf lives.
- Little inter-channel crosstalk: The narrow emission spectra of quantum dots means that there is little overlap between colors, reducing the need for electronic compensation.
- Brightness: Due to very efficient fluorescence generation, quantum dots are often much brighter than traditional fluorophores. This may enable you to detect weakly expressed antigens with a reduced requirement for signal amplification.
- Compatibility: Quantum dots are compatible with other existing organic dyes. In practice, you can add them to an existing experimental set-up without having to redesign everything from scratch.
- Tuneability: By taking advantage of the predictable relationship between quantum dot particle size and emitted fluorescence, customized multicolor assays can be developed. This allows researchers to expand their set-ups beyond off-the-shelf quantum dot preparations.
Drawbacks of Quantum Dots
As with many other things in life and science, quantum dots carry some limitations. Before enthusiastically placing a large order, you should consider the following points when designing FACS experiments with quantum dots.6
- Large Size: Quantum dots are relatively large molecules. Thus, they won’t diffuse across membranes. Any technique used to forcefully permeate the cell membrane for intracellular staining may destroy the cell or lead to artificial results.
- Toxicity: There has been some talk about quantum dot toxicity to cells, mostly due to their essential metal components.7 The good news is that there are cadmium- and heavy metal-free alternatives. Furthermore, surface modifications that reduce their hydrophobic nature to make them work in vivo also reduce their toxicity.8
- Photostability: Whilst photostability is a blessing in most cases, there may be set-ups that require the dye to disappear at a certain point in the experiment. In situations where you cannot wash a particular stain from your cells, quantum dots probably won’t be a feasible option.
- Yield deterioration: When the quantum dot surface quality is low, the ratio of emitted to absorbed energy is unfavorable. This leads to dim signals, or quantum dots starting to blink and becoming invisible. At best, such cases may only be salvageable with highly sensitive detection systems.
Quantum Dots in Your Research
Quantum dots have caused quite a splash in the fluorescence imaging toolbox. With careful experimental design and reagent selection, their many positive attributes should outweigh their potential limitations.
Have you used quantum dots yet? Feel free to get in touch with us by writing in the comments section!
References
- Chan WCW, Nie S. (1998) Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science 281:2016–8.
- Saha A et al. (2013) Near-Unity Quantum Yield in Semiconducting Nanostructures: Structural Understanding Leading to Energy Efficient Applications. J Phys Chem Lett. 4:3544–49.
- Wikipedia. Exciton. [Online] 26 Feb 2017.
- Barts and The London School of Medicine and Dentistry. Blizard Institute of Cell and Molecular Science. Flow Cytometry Core Facility. [Online] 26 Feb 2017.
- Ibáñez-Peral R et al. (2008) Potential Use of Quantum Dots in Flow Cytometry. Int J Mol Sci, pp. 2622–2638.
- Aggeliki, K. Bright Hub Engineering. Quantum Dots Advantages and Disadvantages. [Online] 26 Feb 2017.
- Quantum Dot Weathering Results in Microbial Toxicity. Mahendra, Shaily, et al. 2008, Environ. Sci. Technol., pp. 9424–9430.
- Valizadeh A et al. (2012) Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Research Letters.
You made it to the end—nice work! If you’re the kind of scientist who likes figuring things out without wasting half a day on trial and error, you’ll love our newsletter. Get 3 quick reads a week, packed with hard-won lab wisdom. Join FREE here.