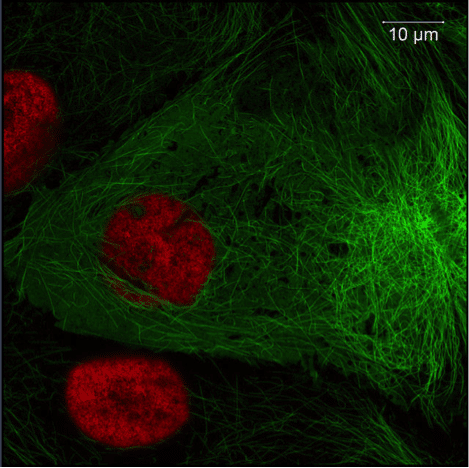
Fluorescent-based microscopy techniques are some of the most common ways to visualize biological structures. Almost any protein- or nucleic acid-based molecule can be tagged with a fluorescent marker or dye and subsequently detected by a light microscope. This fluorescence is seen as a bright object against a black background, allowing for intense and clear images. Therefore, fluorescence microscopy can be a highly sensitive way to perform localization experiments.
Forget the advanced degree
It is important to have a basic working knowledge of the fundamentals of fluorescence in order to successfully image and efficiently troubleshoot your experiments. Fluorescence is a powerful tool; fortunately, its underlying principles are possible to grasp without an advanced degree in physics.
Relieving instability
So what, exactly, IS fluorescence? Simply put, fluorescence is created when a molecule emits light at a certain wavelength after being excited with light at a different wavelength. Molecules that have fluorescent properties are referred to as “fluorophores.” By definition, these molecules are excited by a higher energy—shorter wavelength—of light. This excitation creates instability within the chemical structure of the molecule due to this extra absorbed energy. The molecule will release photons to relieve this instability, which are of lower energy and result in light of a longer wavelength. These emitted photons create the fluorescence that can be detected by a light microscope which has been set to capture the appropriate wavelength of light.
I can see DNA!
Due to their unique molecular structures, each fluorophore is excited by, and also emits, specific wavelengths of light. For example, the commonly used fluorescent DNA probe, ethidium bromide, has an excitation wavelength of ~493 nm and emits light at a wavelength of ~620 nm. In other words, ethidium bromide absorbs energy from blue/green light and emits orange/red light. Subsequently, to visualize DNA which has been stained with ethidium bromide you will need a light microscope which is equipped with a source of blue/green light and with a filter to detect orange/red light.
A fluorescent rainbow
There are almost as many different fluorophores as there are combinations of excitation and emission wavelengths. These fluorophores can be chemical probes (such as ethidium bromide), or they may be contained within proteins (such as green fluorescent protein). Modifications in the laboratory have made it possible to create a whole rainbow of fluorophores, allowing you, the investigator, to design visualization experiments according to your specific questions, goals, and needs.
This series will cover basic theories regarding the following aspects of fluorescence microscopy:
- Fluorophores: history, types, GFP
- Fluorescent proteins vs. fluorescent probes
- Fluorescent resonance energy transfer (FRET)
- Fluorescent recovery after photobleaching (FRAP).
However, if you would like us to cover a specific topic, please let us know!