Can You Stand the Cold? Cryosectioning for Beginners

Before you can perform histology on your tissue samples – you need to prep them. This means you must fix them, embed them, and section them into thin slices for analysis. A great way to slice your tissues is cryosectioning. But cryosectioning is not so great when your tissues melt, fold, curl, wrinkle, tear, or crack while you attempt to section them.
Let me help you eliminate those wrinkles – on your frustrated foreheads and your tissue slices. Below I will teach you how to troubleshoot and avoid these pesky cryosectioning problems.
Tissue Prep
You can perform cryosectioning on both your formalin-fixed and fresh tissue samples. However, formalin fixation better preserves the antigens within your tissue.
Briefly, tissue fixation is your best option for morphological analysis, while using fresh tissue allows you to avoid having your antigens masked by fixation cross-linking. Once you determine the best fixation method for your tissue, then you are ready to move on to preparing your block and freezing it for cryosectioning (see reference links).
When freezing your tissue samples, you should use a rapid freezing technique to avoid ice crystal formation within the tissue (see reference links). Always ensure you know the orientation of your tissue block, particularly if you have multiple tissue samples within one block.
It is crucial for you to mark one spot on your tissue block after mounting it onto the cryostat chuck to know the orientation. The edges of your block on round or square chucks will become indistinguishable once mounted. Now that you have chosen how to prepare and freeze your tissue, you are ready to move on to cryosectioning.
Sectioning in Brief
Place your prepared tissue block within the cryostat chamber (Figure 1) for 30-60 minutes prior to beginning your sectioning, to allow the tissue to acclimate to -200C. You should begin your cryosectioning practice with either non-essential tissue or a block of O.C.T. (Optimal Cutting Temperature compound).
Learning to cryosection with essential tissue will only lead to heartache. So avoid it at all costs. And when sectioning maintains your patience and stay calm. Otherwise, your hot temper may melt your tissue!
Now let’s start sectioning. First thing first, because it is not always intuitive when cryosectioning you advance your tissue by rotating the handle forward (towards the back of the machine), away from you. Start your sectioning practice by sectioning your tissue at a thickness of ~50µm. Then, as you begin to have more success with mounting your tissue without problems, gradually decrease the thickness (40µm, 30µm, 25µm, and 20µm). Tissue folds, tears, and bubbles are common. Minimizing them is essential to have good-quality images and accurate quantification of your histological results. And it takes practice to minimize them.
You should use a fine-tip paintbrush to carefully flip the tissue over so that it will naturally uncurl upward toward the glass plus slide. Then, press your finger onto the glass slide (directly opposing your tissue cord) as you gently press the glass slide down towards the tissue. The warmth from your finger helps the tissue unfold and adhere to the glass slide. While doing this do not press the glass slide all the way down to the metal plate (stage) with too much force, and do not let your glass slide out of your grasp onto the tissue otherwise the tissue will likely freeze and stick to the metal plate.
Optimally you want to keep your tissue block as close to the handle of the anti-roll glass bar as possible. This is because the tissue will begin to curl upwards once you lift the glass. When mounting your tissue to the glass slide, always pay close attention to how close your hands are to the blade – you do not want to cut yourself!
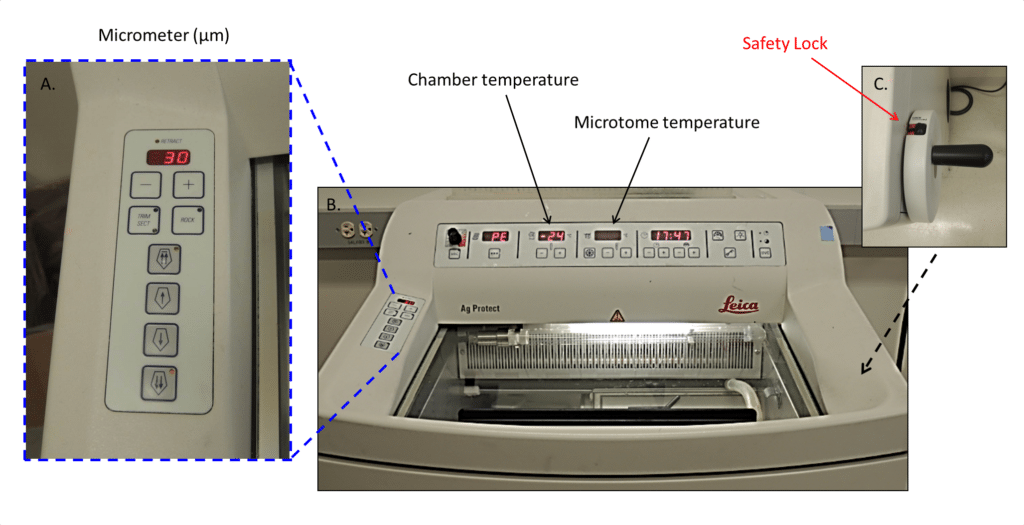
Figure 1: A. This is the micrometer display. This is where you see how thick or thin your slices of tissues are, expressed in µm. B. This is the actual cryostat chamber with visible chamber and microtome temperature settings. C. This is the handle and your all-important safety lock.
Where to Put Your Hands (in Detail)
This sounds silly but if you have never sectioned this is a real consideration. Sectioning is a bit like “patting your head and rubbing your stomach at the same time”. It takes some coordination and practice, but you CAN do it. Just go slowly at first, and be patient with your early bumblings.
- Step 1: With your right hand you will rotate the handle that controls the tissue block movement and cuts the tissue. Simultaneously, with your left hand, you need to press gently on the anti-roll glass.
- Step 2: As your ribbon of sectioned tissues comes off the blade (between the anti-roll glass and the metal plate) pick up a paintbrush with your dominant hand while you lift the anti-roll glass with your non-dominant hand.
- Step 3: Stop sectioning and quickly pick up a slide with your non-dominant hand and use your dominant hand and paintbrush to flip the tissue over. Take the time to correctly orient the tissue before it warms up and unfolds. Gently press the glass slide down towards the tissue and let the tissue adhere up towards the slide as it warms.

Figure 2: A. Picture of the microtome found within the cryostat chamber, with visible spindle, tissue-mounted chuck, anti-roll glass, and metal plate/stage. B. The chuck, pictured here, is what holds your tissue. C. The entire cryostat chamber.
Cleaning Your Cryostat
You will need to clean the cryostat after every session, and likely a few times during. But never clean components inside the chamber with water! If you do the components will ice over and freeze in place, rendering them immobile. To clean, simply wipe down with dry Kim wipes or paper towels to brush tissue and medium off the metal surfaces. And be sure you ONLY use Kim wipes for the glass surfaces and near the blade. Otherwise, you will get fibers on your equipment that can cause fractures in your sections.
Checklist for Success
Before you begin…
– Check that the safety lock is locked on the handle before you manipulate ANYTHING within the chamber. If you do not, you run the risk of possibly slicing your hand.
– Check the micrometer display for your desired tissue thickness (µm).
– Line up your fine-tip paintbrushes and forceps inside the cryostat chamber. This is to keep them cold and give you easy access. This is important because once you get your sectioning groove on you will not want to go rummaging for supplies.
As you section…
Maintain your rostral/caudal orientation and sample # order as you
- make the block in the plastic cryomold (see references at bottom)
- transfer the block from the plastic cryomold to the chuck (see Figure 2B, chuck without tissue)
- mount the chuck into the spindle on the microtome (see Figure 2A, chuck with tissue)
- label glass plus slides, and double-check that your slides are coated (plus glass slides) to ensure good tissue adhesion
It may help if as you go, you write down each of these steps on a piece of paper with the corresponding rostral/caudal orientation and sample number. This will help you to later verify orientation when the inevitable doubt creeps in. This is particularly important to do when you are mounting several tissue samples in a row or column, since depending on what you are sectioning, they can be indistinguishable. Similarly, the edges of the round tissue chucks and the sides of the square tissue chuck all look the same, so putting a dot or line on your O.C.T. block with a sharpie, to give you a reference point is quite useful.
What to Do When Things Go Wrong
And they will! Above all, if any problems arise, stay calm. Troubleshooting is cryosectioning’s middle name. So know it is not just you. If you work on a cryostat and do not have any troubleshooting, you are one in a million!
Folding/Curling Problems
One of the most common problems is tissue folding or curling as it is cut. The first weapon against this is none other than a small artist’s paintbrush. You can use this brush to gently coax the occasional curled tissue flat. But if you have mastered this “art” and are experiencing persistent curling or folding it is time to fix the root of this problem. Most importantly check that your cryostat blade is sharp and not warped. A lot of cryostat frustration stems from dull or warped blades and you should really use a fresh blade every sectioning session. Trust me, a new blade is money well spent.
Smudging/Smashing Problems
If your tissue is coming out smudged or almost smashed (not smooth at all) then you need to check the temperature of the cryostat head. When the tissue is too cold it can smudge and will not be a thin slice. The recommended chamber temperature is -200C. However, if you keep your microtome spindle (which holds the tissue block, Figure 2) at -200C, then you should keep your chamber temperature just slightly colder at about -220C or -230C. A clever way to verify if the tissue/microtome spindle temperature is your problem is to briefly warm it.
The simplest way to do this is to place your gloved finger against the side of the tissue block (the cutting edge) for about 10 seconds. Of course, triple-check that the handle is locked for safety first! Then unlock the handle and make another slice. If the smudging is resolved, then the cryostat head needs to be warmed up a degree or two. And remember, the longer you have the tissue off the metal cryostat plate (beneath the anti-roll glass), and the longer you hold it with the paintbrush or forceps, the quicker it will melt and curl/fold.
Streaking/ Tearing Problems
If you are observing a streak or consistent tear line in your tissue, check your anti-roll glass and tissue blade carefully. Sometimes frozen tissue or O.C.T. is stuck on the anti-roll glass or blade and both can cause streaks. To solve this, just carefully wipe the anti-roll glass with a Kim wipe tissue. If the tear still persists, move your tissue horizontally along your blade, because the blade might be warped in one spot. If you are STILL having problems replace the blade.
Whether you are getting heated over your frozen tissue or just preparing to learn to cryosection, have no worries – just set out a good plan, and a backup plan for troubleshooting, and your newly acquired patience will help keep you from melting your tissue. And remember my quick tips:
- Always remember to use the safety lock when you are adjusting or troubleshooting anything within the chamber.
- Keep a good record of tissue sample numbers within your block and on your slides, because their orientation (rostral/caudal) will change several times during the preparation of the block, mounting the block, and mounting onto the slides.
- Always remember to use plus glass slides to keep your tissue adhered to the glass during histological staining.
- Double-check the micrometer setting on the cryostat.
- Remember to flip your tissue over to allow it to naturally unfold upward towards the warm glass plus slide.
Above all, stay calm and cool so you do not melt your precious tissue sample. If you have any questions don’t hesitate to leave a comment.
Want to know more about histology? Visit the Bitesize Bio Histology Hub for tips and tricks for all your histology experiments.
Resources
- Peters, S.R. (2010) Practical Guide to Frozen Section Technique. Springer Science + Business Media, New York. Accessed April 2023
- See “Tissue Freezing Methods” on the resources page of the Mouse Histology & Phenotyping Laboratory. Accessed April 2023
- Paul, C. (2014) Alternatives to Paraffin: Cryo and Resin Embedding and Sectioning for Histology, Bitesize Bio.
2 Comments
Leave a Comment
You must be logged in to post a comment.
What are your thoughts about having tissue commonly stuck to the anti-roll glass plate? I am learning so I am not sure whether I am just a terrible at sectioning or if something else is going on. Even after spraying with cytocool, the tissue commonly sticks. Thanks for your input in advance, I really enjoy your blog.
Jordan, your anti-roll plate is probably too warm if the tissue sticks to it. Turn it down onto the knife and close the lid to the cryostat, then go and have a cup of coffee while the whole thing cools down.