Ever done a multicolour flow cytometry experiment, run all your controls, done your compensation and then started to analyse your data and realised that you can’t work out where to put your gates?
To err is human…
When acquiring data on a cytometer, there can be measurement errors due to counting statistics, errors in processing the data and inaccuracies of log amplifiers. When data is compensated these errors are revealed and there will be spreading of your data. The more photons that are counted (the cells get brighter) so the measurement error increases. This causes all the channels to spread when correctly compensated but we notice it more on the brighter populations.
You should be aware of these errors and in order to minimise the problems of spreading of your data, you can perhaps design a better experiment, or avoid channels that will have large spreading of data. However, make sure you have the correct controls to make sense of your data and interpret it correctly.
Fluorescence minus one controls
Fluorescence Minus One (FMO) controls are now commonly used in multicolour experiments and they must be run with your experiment. These controls are cells (same cell type as your sample) stained with all the fluorochromes minus one fluorochrome. This allows you to take into account any spread of fluorochrome(s) into the unlabelled channel and allow you to put your gate in the correct place. They are essentially your gating controls for your experiment.
Enjoying this article? Get hard-won lab wisdom like this delivered to your inbox 3x a week.

Join over 65,000 fellow researchers saving time, reducing stress, and seeing their experiments succeed. Unsubscribe anytime.
Next issue goes out tomorrow; don’t miss it.
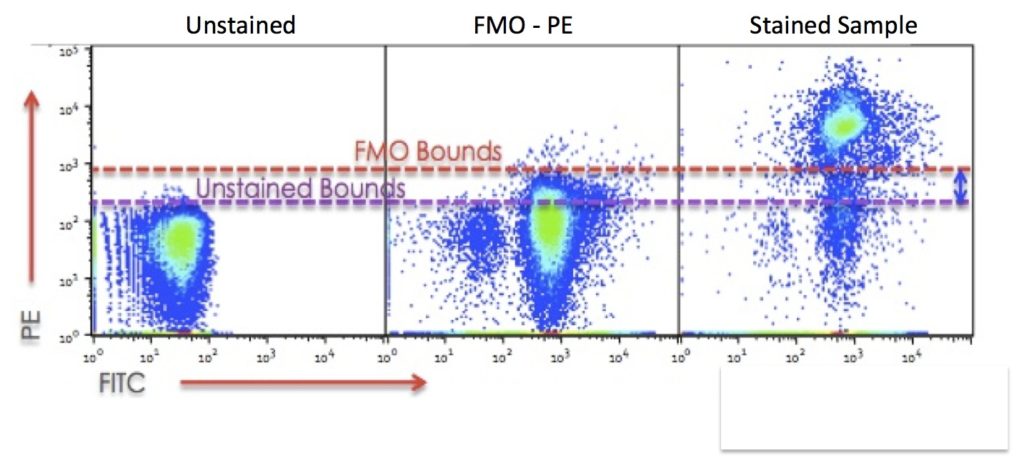
Example Data:
This data shows FITC and PE staining from the panel and displays the accurate identification of the CD4 (PE+) T cell population using the FMO control. The plots show that even though you can’t see the PE-Cy5 and PE-Cy7 data, the compensation between the channels reveals the spread of data in the PE channel. Therefore, in order to accurately gate the PE+ cells the gate should be placed at the top of the FMO staining and not the unstained control.
Are they really necessary?
Yes, they are a great control and you will find more and more reviewers asking for them. However, you might not need them for all channels. FMO controls are only crucial when accurate identification between a positive and negative population is vital, e.g. when you have dimly expressed antigens or a continuous distribution of staining. Look at your panel, and see which channels need an FMO. It might be worth trying FMO controls for all samples once and then deciding which ones you want to look every time you do your experiment.
Golden rules : Always use controls in your experiments and it is better to run them at the time of your experiment than to have to repeat the whole experiment because of reviewers asking for them.
Data shown from Mario Roederer, NIH Vaccine Centre.
You made it to the end—nice work! If you’re the kind of scientist who likes figuring things out without wasting half a day on trial and error, you’ll love our newsletter. Get 3 quick reads a week, packed with hard-won lab wisdom. Join FREE here.