Flow cytometry remains unparalleled as a single-cell analysis technology. The ability to analyze 14 or more fluorescent parameters on a million cells or more allows for detailed understanding of complex biological processes.
The Problem With Traditional Flow Cytometry
One limitation of flow cytometry is the reliance on fluorescent tags. Even with careful panel design, loss of resolution occurs because issues including autofluorescence and spectral spillover cannot easily be avoided. The greater the number of fluorochromes used, the larger these problems become magnified.
To overcome this issue, a new method of detecting antibodies bound to cells must be considered (CyTOF). This method needs to be robust enough to measure the amount of each label, and distinguish the different labels with sufficient accuracy.
The Solution
One such detection method is a time of flight mass spectrometer (TOF-MS). TOF-MS is a very accurate and robust method for measuring the mass to charge ratio of an ion. In TOF analysis, all ions are accelerated through an electric field of a known strength, and the time it takes these ions to reach the detector over a known distance is measured. The heavier the ion, the longer it takes to get to the detector.
In mass cytometry, the label of choice is highly-purified, stable isotopes. Currently the lanthanide series is used, but additional isotopes will become available as the conjugation chemistries are optimized.
How CyTOF Works
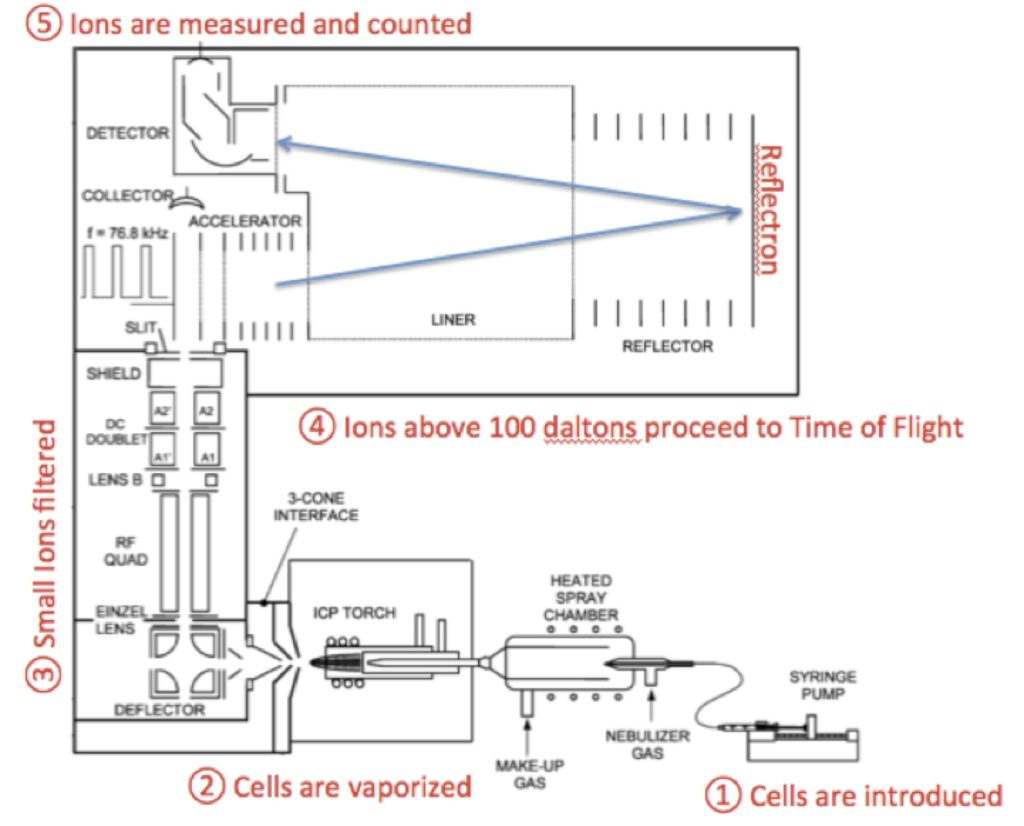
To perform mass cytometry, the isotopes need to be introduced into the TOF without also contaminating the instrument with cells. This requires the vaporization of the cells, leaving only the isotopes. To do this, the cells are introduced to an inductively coupled plasma (ICP); here the cells are vaporized and the metal ions are introduced into the TOF.
Taken together – the labeling of antibodies with stable isotopes, the vaporization of cells by the ICP and the detection of the ions by TOF-MS are pulled together in the DVS CyTOF mass cytometer. Schematically, this looks like such:
The Pros and Cons of CyTOF
The advantages of the CyTOF include:
- Traditional labeling techniques can be used – minimal change to current protocols
- No ‘autofluorescent’ equivalent in mass cytometry – cells do not contain lanthanide ions inside
- Minimal/no ‘compensation’ needed – although there is some well defined oxidation that can be readily addressed
- Panel design is much easier
Limitations of the CyTOF include:
- Slower acquisition compared to traditional flow cytometry – maximum of about 1,000 events per second
- Sample prep must be cleaner than traditional flow cytometry – all biological material is vaporized and can lead to carbon buildup and reduce sensitivity
- Limited (but growing) catalog of commercially labeled antibodies
- Complex data structure requires new methods of data analytical tools
- No equivalent FSC and SSC measurements – cells are vaporized
Mass cytometry represents a new way of measuring and phenotyping cells. It takes what is familiar (labeling cells) and extends the dimensionality of the data to over twice as many parameters as that of traditional fluorescent flow cytometry. This can be a very powerful tool for screening multiple intracellular targets in well-defined phenotypic populations, such as was shown in the paper by Bendall et al (2011).
If screening very complex populations is critical for your research, consider investing in learning and utilizing this technology – if not at your institution – reach out to an institution that has one. Since the cells must be fixed, it is possible to mail samples to another institution for analysis.
Originally published on April 22, 2014. Updated and revised on July 6, 2016.