Many biological applications that use cells, such as microbiology, cell culture, and blood work, require that we determine cell concentration for our experiments.
Cell counting is actually quite straightforward and requires a counting chamber called a hemocytometer, a device invented by the 19th-century French anatomist Louis-Charles Malassez to perform blood cell counts.
A hemocytometer consists of a thick glass microscope slide with a grid of perpendicular lines etched in the middle. The grid has specified dimensions so that the area covered by the lines is known, which makes it possible to count the number of cells in a specific volume of solution.
The most common type of hemocytometer has an “H” shape engraved in the middle that encloses two separate mirror-like polished grid surfaces and provides the coverslip mounting area (Figure 1).

Here, we’ll talk you through using a hemocytometer and calculating your cell concentrations accurately.
Using a Hemocytometer in Four Simple Steps
1. Dilute Your Sample with Trypan blue
Trypan blue is a stain that allows you to distinguish dead cells from living cells. When mixed with your cell sample, any dead cells will be stained blue by the dye, meaning that you can count only those cells that are living and viable.
You can dilute your sample with trypan blue at any ratio, but a 1:1 ratio is the most common. Whatever dilution you use, make sure to note it down as you’ll need this for your final calculation.
2. Loading the Hemocytometer
Before you get started, ensure that both the hemocytometer and its coverslip are clean by removing any dust particles with lens paper. Coverslips used for mounting on hemocytometers are specially made to be thicker than conventional microscopy coverslips because they must be able to overcome the surface tension of a drop of liquid.
Make sure you place the coverslip over the counting surface before loading the cell suspension. Then place the pipette tip with your sample into one of the V-shaped wells, and gently expel the sample. The area under the coverslip fills by capillary action.
Enough liquid should be introduced so that the mirrored surface is just covered, usually around 10 µl, but don’t overfill the surface. You can load two samples on one hemocytometer, one into each of the two grids.
The loaded hemocytometer is then placed on the microscope stage and the counting grid is brought into focus at low power. Allow the sample to settle for a couple of minutes and avoid moving the coverslip as it might introduce air bubbles and make counting difficult.
3. Counting Cells in a Hemocytometer
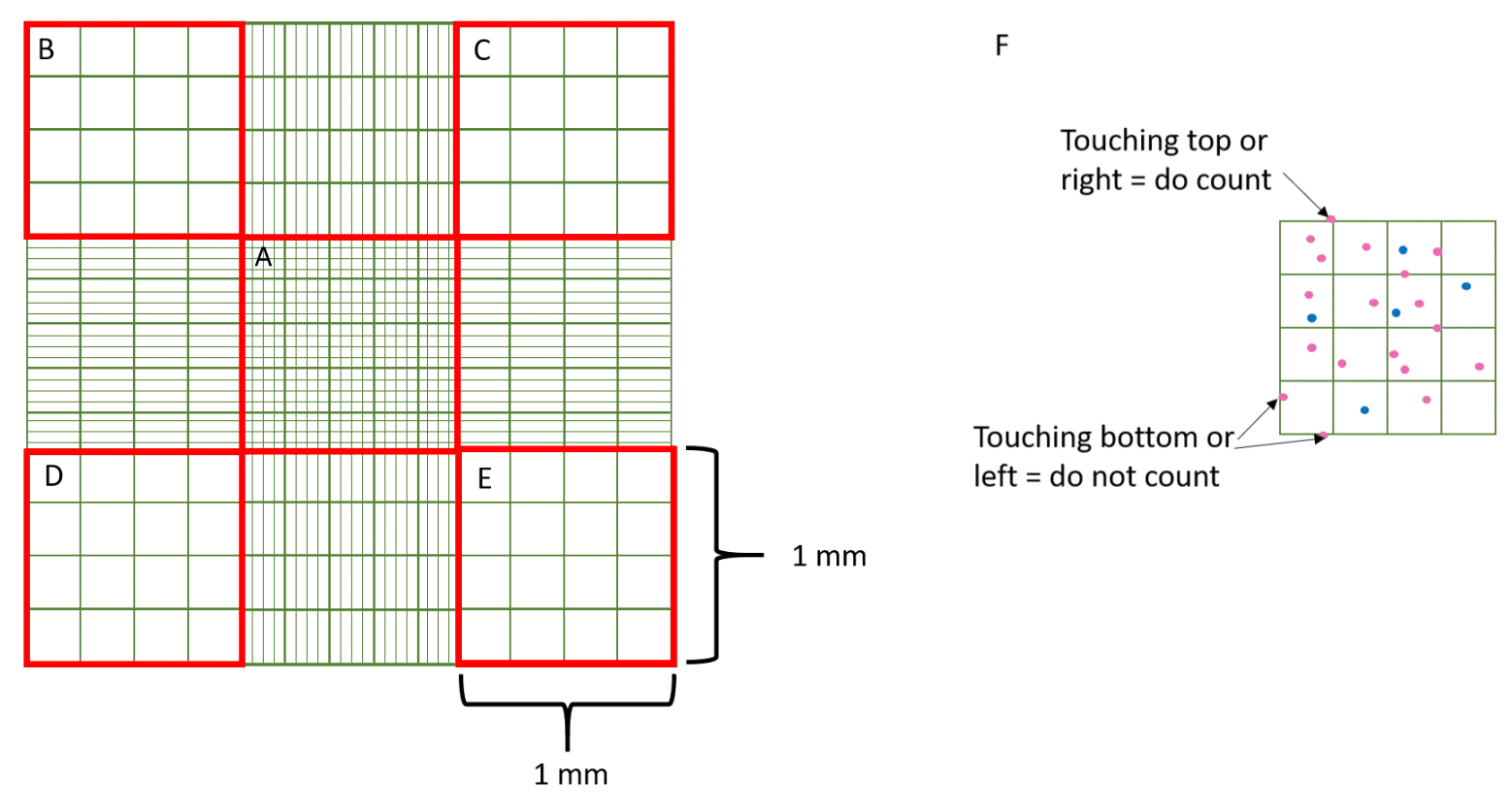
The full grid on a hemocytometer contains nine squares, each of which is 1 mm2 (Figure 2). The central counting area of the hemocytometer contains 25 large squares and each large square has 16 smaller squares.
When counting cells that overlap an exterior line or ruling, count only those cells on the top or right-hand line of the large square to avoid counting cells twice. Suspensions should be dilute enough so that the cells or other particles do not overlap each other on the grid, and should be uniformly distributed.
To perform the count, determine the magnification needed to recognize the desired cell type and systematically count the cells in selected squares so that the total count is approximately 100 cells, a minimum number of cells needed for a statistically significant count.
For large cells, you can simply count the cells inside the four large corner squares (Figure 3B–E) and the middle square (Figure 3A). For a dense suspension of small cells, you may wish to count the cells in the four outer and middle squares of the central square (Figure 3A) or make a more dilute suspension.
Remember if a cell overlaps a line, count it as “in” if it overlaps the top or right-hand line and “out” if it overlaps the bottom or left-hand line (Figure 3F).
The area of the middle (Figure 3A) and each corner square (Figure 3B–E) is 1 mm x 1 mm = 1 mm2. The depth of each square is 0.1 mm. Hence, the final volume of each square at that depth is 100 nl.
4. Calculating Cell Concentration
You can calculate your cell concentration using the following formula:
Total cells/ml = (Total cells counted x Dilution factor x 10,000 cells/ml)/ Number of squares counted
So, for example, if you diluted your sample 1:1 with Trypan blue (dilution factor is 2 in this case), and you counted 325 cells in the four corner squares plus the central big square (number of squares counted is 5), then:
Total cells/ml = (325 cells x 2 x 10,000 cells/ml)/ 5 = 130 x 104 cells/ml
If you want to know how many cells you have in your original sample, just multiply the cell concentration by the total sample volume. For example, if your original sample volume is 5 ml, then:
Total cells in sample = 130 x 104 cells/ml x 5 ml = 650 x 104 cells
That’s it! Once you understand the basics of using the hemocytometer, cell counting really is as easy as 1, 2, 3! We’d love to hear any of your tips for cell counting; drop us a line in the comments.
Why not brighten up your cell culture room and display some helpful pointers for all those busy users who come and go? Download our handy (and free) cell culture posters today.
Originally published 2013; updated and republished June 2021.





