The presence of supercoiled plasmid DNA on a gel can be inconvenient for molecular biologists, especially beginners because it is easy to misinterpret. But what is supercoiled DNA? And why does it occur? In this article, we’ll talk about how to recognize supercoiled DNA on a gel and how to keep it from derailing your experiments.
What Is Supercoiled DNA?
Consider the following example. An older style ground line telephone cord is traditionally a coiled structure. Frequently, owners of these ancient communication devices would become frustrated due to the already coiled cord becoming even more tightly coiled and, therefore, tangled and compacted. Anyone who has tried to walk across the room while talking on a phone with a tangled cord knows that there is often not enough slack or length in the cord to keep the phone from falling off the desk.
DNA is capable of doing this as well because it is also a coil – a double helix. The double helix can be wound even tighter than its normal tension of 10.5 base pairs per turn to create a more compact form known as a supercoil. For a visual demonstration, watch this video of a rope being supercoiled.
The purpose of supercoiling in nature is to compact DNA, which is typically very long and bulky, into more densely packed and functionally “shorter” strands for enclosing in the nuclei of cells. Enzymes called topoisomerases are capable of winding and unwinding the supercoil for various biological processes. Plasmids are also susceptible to supercoiling, which presents a common technical challenge in molecular biology.
Supercoiled plasmid DNA runs as three bands of distinctly different sizes on an agarose gel (see an example below). If you don’t know what to look for, you may interpret these results as three different restriction fragments. Even more frustrating: one of these three bands is often fainter than the other two, so if you load a smaller amount of DNA on the gel, you may only see two bands. In both circumstances, it is easy to mistake these different species of uncut plasmid for linear fragments and misinterpret your results accordingly.
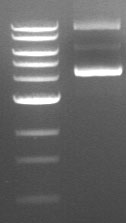
Why Am I Seeing Supercoiled DNA on My Gel?
Supercoiling is not necessarily caused by errors in technique; rather, it is an inherent property of DNA. If you are seeing supercoiled DNA on your gel after performing a restriction digest, there are a few possible explanations. The simplest explanation is that the restriction enzyme you used does not have a recognition site in the plasmid—this means that all you will see on your gel is uncut (circular) plasmid. Alternatively, the restriction site may be obscured due to the degree of folding and coiling of the plasmid. This will result in some amount of uncut plasmid on your gel. The efficiency of your restriction digest will also vary depending on the restriction enzyme you used, since different enzymes cut supercoiled DNA with different efficiencies.
How Do I Contend with Supercoiling?
While you can’t prevent it, there are measures you can take to prevent misinterpreting your results. If you suspect that an enzyme is not cutting efficiently due to supercoiled structure in the region of the restriction site, try using a different enzyme for screening. For example, a gel from an EcoRI restriction digest that is confusing to interpret due to supercoiling may provide more useful results if digested with HindIII. Another possible solution is to use a higher concentration of restriction enzyme in your digest, or increase the digestion time. Check the NEB catalog for enzymes which are particularly sensitive to supercoiling.
Supercoiled DNA Summarized
The best way to prevent supercoiled plasmid from causing you to misinterpret your results is to always include both positive and negative controls. The positive control would be a sample of plasmid that you know cuts reliably with your chosen restriction enzyme, and yields bands of predictable sizes. The negative control would be a mock digest containing no restriction enzyme, which shows you what your product looks like uncut. Multiple bands in this lane indicate the degree of supercoiling in your plasmid template, and allow you to disregard bands of comparable sizes in other lanes.
Have you ever encountered supercoiled DNA in your cloning experiments? How did you handle it?