I was browsing a certain website the other day when I came across a protocol that advised: “When agarose starts to cool, it undergoes what is known as polymerization”
Polymerising agarose? Someone is getting their gel matrices mixed up, and it’s not the first time I have heard this said, so it looks like some myth-busting is required.
So hunker down for a quick run down on the difference between polymerising and non-polymerising gel matrices.
Of the common gel matrices used in molecular biology, polyacrylamide, agar and agarose, polyacrylamide is the one that polymerises. I’ll deal with agarose and agar later.
Polyacrylamide gel polymerisation
Polyacrylamide, used mainly for SDS-PAGE, is a matrix formed from monomers of acrylamide and bis-acrylamide. It’s strengths are that is it chemically inert – so won’t interact with proteins as they pass through – and that it can easily and reproducibly be made with different pore sizes to produce gels with different separation properties.
The polymerisation reaction, shown in the diagram below, is a vinyl addition catalysed by free radicals. The reaction is initiated by TEMED, which induces free radical formation from ammonium persulphate (APS). The free radicals transfer electrons to the acrylamide/bisacrylamide monomers, radicalizing them and causing them to react with each other to form the polyacrylamide chain.
In the absence of bis-acrylamide, the acrylamide would polymerise into long strands, not a porous gel. But as the diagram shows, bis-acrylamide cross-links the acrylamide chains and this is what gives rise to the formation of the porous gel matrix. The amount of crosslinking, and therefore the pore size and consequent separation properties of the gel can be controlled by varying the ratio of acrylamide to bis-acrylamide.
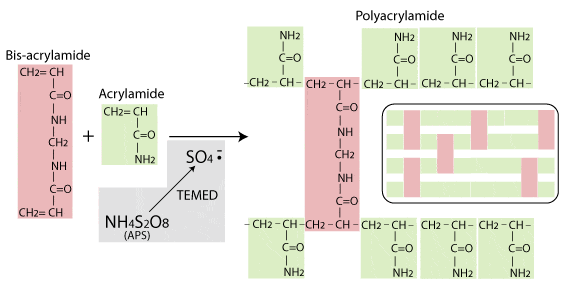
For more information on polyacrylamide gel polymerisation see Biorad Bulletin 1156
Agarose gel formation
So what about agarose? Well, agarose – the main component of the gelatinous agar that can be isolated from certain species of seaweed – is itself a polymer. But, polymerisation is not the mechanism for agarose gel formation.
Chemically, agarose is a polysaccharide, whose monomeric unit is a disaccharide of D-galactose and 3,6-anhydro-L-galactopyranose which is shown in the diagram below.
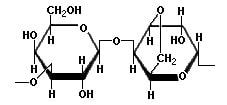
In aqueous solutions below 35°C these polymer strands are held together in a porous gel structure by non-covalent interactions like hydrogen bonds and electrostatic interactions. Heating the solution breaks these non-covalent interactions and separates the strands. Then as the solution cools, these non-covalent interactions are re-established and the gel forms.
So agarose (and agar) gels form by gellation through hydrogen bonding and electrostatic interactions, not through polymerisation