You know the feeling. An assay that performed beautifully last week suddenly behaves differently today. When results shift, it often comes down to details in experimental design and how well your reagents align with your target and assay conditions.
Whether you work with polyclonal, monoclonal, or recombinant antibodies, understanding how they are generated and how they interact with your antigen helps you make more informed choices.
In this article, we will explore experimental optimization with recombinant antibodies and epitope mapping to provide you with the tools to strengthen your experimental design and improve assay performance.
Choosing The Right Antibody for Your Experiment
Antibodies are one of the most widely used reagents in biomedical research, your indispensable tools for protein detection. You use them for Western blots, ELISAs, immunofluorescence, flow cytometry, IHC, and more—essentially every protein detection method in your lab.
How an antibody is produced directly impacts its specificity and reproducibility. Therefore, selecting the right antibody is crucial for your research.
For decades, you only had two main antibody options:
- Polyclonal antibodies
- Monoclonal antibodies
Polyclonal antibodies come from immunized animal serum and are a mixture of antibodies that bind different epitopes on your target protein. This diversity can be valuable for detecting rare or modified proteins, however, each batch is different, so your results can vary when changing between lot numbers.
Monoclonal antibodies are derived from a single B cell clone that is usually immortalized in a hybridoma cell line. They recognize a single epitope, demonstrating high specificity and experimental accuracy.
Despite this, hybridomas are living cells. They can mutate over time, causing genetic drift that changes the antibody sequence and binding characteristics. So whilst monoclonal antibodies improve reproducibility, they don’t eliminate batch-to-batch variability.
This challenge led to the next evolution in antibody technology: the development of recombinant antibodies.
During development, the genes for heavy and light chains are cloned into expression vectors to produce recombinant antibodies. You then express them in host cells such as HEK293 or CHO lines.
Recombinant engineering gives you complete genetic control. Every production run yields the same molecule. No hybridoma drift. No batch variation. No ongoing animal use. Reproducibility is, therefore, at the forefront of recombinant antibody biology.
How Experimental Optimization with Recombinant Antibodies and Epitope Mapping Improves Assay Performance
Understanding the advantages of recombinant manufacturing reveals why recombinant antibodies are the gold standard for research reproducibility and are shaping the future of therapeutic development:
- Batch-to-batch consistency: The same DNA sequence gives the same antibody every time, without biological variability.
- Defined composition: You get a truly homogenous antibody population without genetic drift.
- Scalable production: Once you establish your clone, you can produce recombinant antibodies endlessly, and at any scale you need.
- Custom engineering: You can modify fragment crystallizable (Fc) regions (the constant, non-antigen binding part of an antibody) or add tags to optimize antibodies for your specific assay.
- Animal-free production: You avoid ethical concerns and reduce animal usage in research.
Recombinant Antibodies are Engineered for Reliable Experiments
Recombinant systems are not just a step up from monoclonal antibodies, they unlock entirely new capabilities. Recombinant development enables antibodies to be engineered with defined properties that optimize performance for distinct experimental needs.
One example is FcZero™ antibodies, a Proteintech-engineered recombinant backbone that eliminates Fc receptor binding. The engineering behind these antibodies allows:
Enhanced Reproducibility in Flow Cytometry
In flow cytometry, non-specific Fc receptor binding poses a significant challenge, as it can lead to high background fluorescence and false-positive results. Non-specific binding can be particularly problematic when assessing samples that contain high proportions of Fc receptor-expressing cells, such as macrophages, dendritic cells, and B cells.
Through targeted Fc mutations, Proteintech’s FcZero™ recombinant antibodies solve this problem (Figure 1). By mutating the Fc receptor binding site, these antibodies eliminate non-specific interactions with immune cells and streamline workflows by removing the need for Fc blocking. The result is reduced background noise, improved accuracy and reproducibility of your flow cytometry experiments.
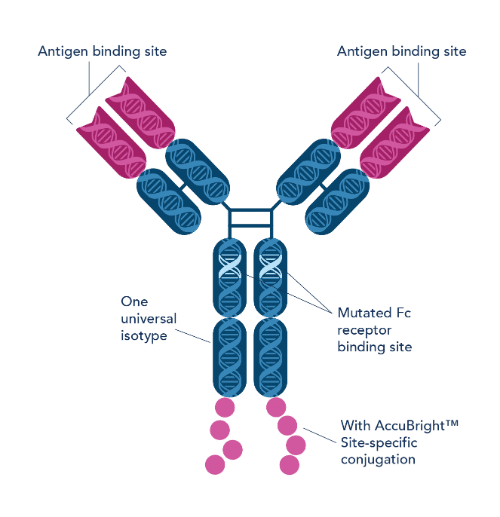
Figure 1. Illustration of Fc-engineered recombinant antibody, FcZero™.
Faster, Sensitive ELISAs
Sandwich ELISAs use matched antibody pairs to increase sensitivity and specificity compared to a direct ELISA. Speedy One-Step ELISA Kits leverage FcZero™ technology for accelerated sandwich ELISA protocols, combined with unparalleled precision and sensitivity.
Optimal Antibody Selection with Epitope Mapping
Knowing exactly where an antibody binds, its epitope, is critical for experimental design.
Epitope information helps you:
- Prevent steric clashes in sandwich assays
- Target specific isoforms or post-translational modifications
- Avoid inaccessibility because of protein conformation or cell architecture
- Selectively activate or inhibit a pathway for therapeutic development
Despite its importance, epitope data are often overlooked and remain largely absent from most commercial antibody catalogs.
Understanding where and how antibodies bind is key to selecting reagents that truly work together. Tools that visualize epitopes in 3D and integrate immunogen selection data help you to identify antibodies targeting distinct, complementary binding sites.
Proteintech’s 3D Epitope Viewer lets you explore antibody binding sites within full 3D protein structures for a clearer picture of antibody–antigen interactions. Alongside this, their immunogen selector makes it simple to pinpoint antibodies that target the exact regions you need.
Together, these resources bridge the gap between structural insight and experimental design. Epitope mapping, which integrates structural, computational and fragment-based approaches, powers these visualization tools.
However, with antibody catalogs expanding every year, finding the right clone can feel overwhelming.
AI-powered search tools are changing that. Platforms like Proteintech’s Able AI streamline the antibody selection process by combining citation, validation, and product information to recommend antibodies matching your specific experimental context. It’s part of a broader shift toward data-driven reagent selection, where algorithms complement your expertise.
Making Reproducibility Routine
Recombinant antibodies are now the gold standard for reproducible, ethical, and data-driven research. This convergence of recombinant engineering, structural insights, and artificial intelligence makes it faster and easier for scientists to find reliable tools for their research.
Whether you’re troubleshooting Western blots, planning a multiplex flow panel, or designing diagnostic assays, understanding how recombinant antibodies work helps you make more informed experimental choices.
The recombinant revolution isn’t just about new technology. It represents a move toward experimental optimization with recombinant antibodies and epitope mapping, giving you the clarity and control you need to design timely experiments that deliver reproducible results.
Learn more about Proteintech’s 3D Epitope viewer, or ask Able to help you find the right product for your experiment.








