Have you ever accidentally forgotten to add the Kozak consensus sequence to the start of a coding gene? Or forgotten to include the stop codon? Did you clone something, then realize you wanted to tag it with something? Or do you want to add restriction enzymes to your PCR product to make it easier to clone into a plasmid? Overhang PCR may be your answer!
In this article, we will discuss what it is, its benefits, and how to do overhang PCR in the lab.
What Is Overhang PCR?
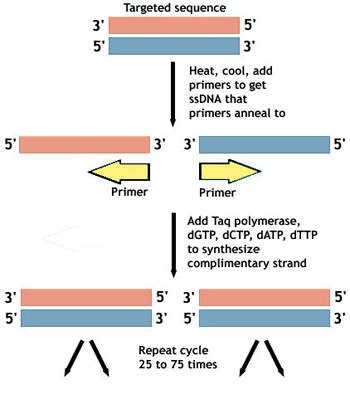
Overhang PCR, also called primer extension PCR, is a technique that utilizes the intrinsic fidelity of the 3’ end of primers for a specific sequence to enable you to add an extra sequence to the 5’ end. This allows you to use PCR to amplify a sequence whilst adding nucleotides to either the 5’ or 3’ ends of the sequence which can then be cloned into a vector backbone for further use.
How Does It Work?
Primers can be designed that have additional “overhang” sequences at the 3’ ends that will then be incorporated into the PCR product. The first cycle of the PCR program causes the primers to anneal to the template at the complementary sites on the primers and create a product that contains the desired overhang regions. Subsequent cycles then amplify this strand of DNA to give a pool of PCR product that contains the new DNA sequence.
How to Add Missing DNA Sequences Using Primers
1. Primer Design
Ideally, at least half of your primer should encompass your existing sequence (although I have gone down to as little as a third and had success), to ensure that the 3’ end of the primer can bind to your target sequence. The rest of your primer can be your overhang. You want to aim for primers about 25 base pairs in length, but it depends on how big your desired overhang is (my largest primers were over 100 base pairs).
Note: If you are using restriction enzymes to digest your PCR product, you will need to add the corresponding cut site in order for it to work efficiently.
Regarding thermodynamic properties, you only need to calculate and ensure you have satisfactory melting temperatures for the portion of the primer that anneals to your template. The manufacturer’s instructions for your polymerase should tell you what the optimal melting temperatures for primers should be.
Do not include the overhang in your melting point calculations, as the first and second cycles are the most important, and subsequent cycles will amplify the entire transcript.
2. Setup and PCR Conditions
Follow the manufacturer’s instructions for your favorite proofreading enzyme to set up your reaction. I find adding DMSO greatly improves the ability of a PCR reaction to succeed, especially when using potential supercoiled templates such as plasmids or genomic DNA.
For the PCR conditions, program the thermocycler as suggested, calculating the annealing temperature from the portion of the primer that anneals to the template, not the entire primer. Visualize the reaction on an appropriate percentage agarose gel. If successful, continue to step 3.
As with most procedures in the lab, the PCR reaction may not be successful with your initial settings, and you may need to optimize the reaction conditions. I suggest running multiple PCR reactions with annealing temperatures both above and below your initial temperature. If you still cannot obtain a PCR product, changing polymerases may help, as each polymerase has different buffer compositions and kinetics, meaning another polymerase may be more amenable to your PCR reaction.
However, it is impossible to predict which ones will work. So I tend to keep the trial polymerase mixes that suppliers send for this purpose.
3. PCR Product
Once you have successfully amplified your PCR product, excise the correct band from the agarose gel and gel purify it using your lab’s methodology or columns. This PCR product can now be ligated into a vector, whether it be digested with restriction enzymes that have been engineered into the overhang or poly-A-tailed and T/A cloned.
Once you have successfully cloned your PCR product into your plasmid of choice, I strongly recommend that you sequence your plasmid to ensure the overhangs are correct and present!
Overhang PCR in Summary
Overhang PCR is a great way to add extra DNA sequences into clones that you may have forgotten at an earlier step. It’s definitely a useful method to have in your arsenal.
Like all PCR methods, take care when designing your primers and calculating their melting points, and be careful with your primer concentration, annealing temperatures, and extension times.
Happy Cloning!
Originally published December 2014. Revised and updated May 2023.