Setting up a Fermentation or Perpetuum Mobile Cell Culture

Some names are confusing. For example, ant-lion is not an ant – or a lion. Likewise, fermentation in the scientific sense does not involve using a ferment or brewing beer. In science, fermentation is the setting up of a long-term culture of eukaryotic or prokaryotic cells.
Fermentation is invaluable in providing a steady flow of cell-derived standard products such as:
- Cell biomass
- Cellular components
- Extracellular metabolites
- Modified substrates
Fermentation – Batch Cultures
The type of culture most frequently used in lab fermentations is a batch culture. Batch culture serves well for short-term experiments, but you can be mislead if you extrapolate results of batch cultures to long-term studies, since batch cultures contain mixed cell populations because of a continuously changing environment (e.g. nutrient depletion, aging cells, metabolic byproducts). Certain cases e.g. the study of competition between different microbial strains, are better assessed under steady-state conditions.
To Carry Out Batch Culture:
- You inoculate a starter culture into fresh medium in a small container, such as a flask.
- If you allow it, your cells go through the S-shaped curve of growth stages:
- After inoculation, the culture goes through a rather non-eventful lag phase where little happens, as cells adjust to their environment and recalibrate gene expression.
- The culture then enters a period of exponential growth, known as the log phase. During this phase, most cells in the population are viable and actively growing and dividing.
- The resources necessary for growth will become depleted relatively quickly, and this usually happens cells while cells are in the stationary phase, which is followed by the death phase as cells die off.
Fed-Batch Fermenation:
Nowadays, fermentation is used for large-scale monoclonal antibody production in the rapidly growing field of monoclonal antibody therapy. Using highly efficient setups, immortalized hybridomas for production of monoclonal antibodies in large quantities that are readily purified through centrifugation, filtration, and chromatography. Full-length antibodies are only part of in vitro monoclonal antibody production, and some clinical applications may only require a partial antibody. Fragments representing different aspects of antibody structure e.g., a variable region with or without a constant region are easier to make than full-length antibodies, can be made with cheaper microbial expressions systems.
Fed-batch is a deviation from a batch culture whereby nutrients are fed to the bioreactor throughout the culture period, while the product remains in the bioreactor until the end of the run. The additional nutrients circumvent the nutrient depletion that occurs in the traditional batch culture. There are a number of other situations where fed-batch setups are useful. These situations typically involve controlling nutrient levels to avoid growth inhibition of the cultured cells.
Why Set Up a Chemostat Culture?
What happens if you keep supplying fresh medium to the culture (feed) and removing growth waste products (effluent) in a vessel that allows you to do it? You will have a chemostat or a fermentor culture.
- Chemostat culture is the first choice in a number of situations, e.g., you may want to study processes that happen in actively growing cells, and have an unlimited supply of cells that don’t need to be replenished frequently.
- Alternatively, you may wish to have large quantities of a certain product (e.g. a secreted or non-secreted protein) produced by your cells, requiring large culture volumes over relatively long time periods. It is easier to culture cells under constant conditions in a chemostat culture than it is to grow cells in batches.
Many commercial biotechnological products are obtained through chemostat cultures in large fermenters, e.g., the antibiotic monensin and the statin lovastatin (1, 2).
If your institution has one of the many commercially available fermenters, you can make you own chemostat culture using the following setup:
1) Preparation
- Because your culture will be growing for a long time, take extra precaution to ensure sterility throughout. Disinfect all parts of your fermentor and ensure that your starter culture is not contaminated.
- Ensure that you have enough medium for the whole duration of the fermentation, and always have more than what you expect to use.
2) Perpetuum Mobile Culture
In the beginning, your culture will have a lower density than the maximum amount of cells that can be maintained according to the limiting factor – usually carbon or nitrogen. This means that cells will happily enter log phase. When the cell concentration exceeds the maximum sustainable density the cells will stop growing. The output valve will remove cell excess. This will lower the cell density and the remaining cells will start to divide again.
The culture density will oscillate with diminishing amplitude until it reaches a steady state. Once in a steady state, the fermentation can proceed for months if you supply the fermentor with enough medium and avoid contamination.
3) How to Calculate Dilution Rate
To calculate how fast you need to remove and add fresh medium to the chemostat, you need to know the following:
*Medium flow rate (M) – volume of medium removing per amount of time, L/h
*Volume of the culture (V), l
You can calculate the maximum dilution rate (how fast you can remove the cells) using:
*µmax – maximum growth rate;
*S – nutrient concentration;
*Ks – Michaelis-Menten constant of the cell growth;
From experience, the chemostat culture requires a lot of media and equipment preparation – sterilizing everything is essential. The first run will also require adjustments of the flow rates of the media input and waste outflow. But once you get the optimal parameters you can sit back and relax becoming a much in demand expert on perpetuum mobile culture.
Literature:
- Parekh, S., Vinci, V. A., Strobel, R.J. (2000). Improvement of microbial strains and fermentation processes. Applied Microbiology and Biotechnology Volume 54 (3) pp 287–301
- Ziv, N., Brandt, N.J., Gresham, D. (2013).The Use of Chemostats in Microbial Systems Biology. J Vis Exp. 14 (80)
- Basic Chemostat Parameter Calculations (2014). University of Plymouth, Lecture for MBIO208 Microbial Life
1 Comments
Leave a Comment
You must be logged in to post a comment.
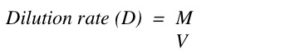
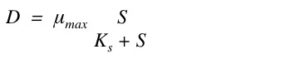
[…] because most of the cells are at optimum health. You can maintain log phase for a long time in a chemostat culture. But in normal, batch conditions, sooner or later the cells run out of a limiting factor and […]