An Introduction to MassTag PCR
New ways to perform PCR emerge all the time. This speaks for the speed of technological advances, and reflects the ongoing need to keep up with fast-moving research.
We all know that PCR’s main purpose is to amplify a stretch of nucleic acids based on sequence-specific primers. Nowadays, a wide range of PCR techniques exist, including qPCR, RACE-PCR, Digital PCR, Multiplex PCR, MassTag PCR and many many more.
In this article, I will give you an overview on MassTag PCR and how you can use it in your research!
What is MassTag PCR?
MassTag PCR is a type of multiplex PCR that uses mass spectrometry to distinguish between PCR products. To recap briefly, multiplex PCR allows you to detect multiple targets simultaneously in one PCR. MassTag PCR is extremely useful with limited sample material and when you need a high degree of accuracy in quantitation. Using this technique, you avoid a common source of error in standard qPCR, namely well-to-well variation.
Multiplexing with MassTag PCR
In MassTag PCR, the presence of a particular tag (i.e., a MassTag) on each PCR product serves as a ‘barcode’ for that specific amplicon. Mass spectrometry separates and counts these barcodes after completion of the PCR.
Incredibly, MassTag PCR can distinguish PCR products that differ by as little as one nucleotide. Because there is theoretically no limit to how many products can be distinguished by mass spectrometry, you can investigate a large number of targets in one PCR. This is extremely useful in many applications where sample material is limited, such as infectious disease diagnostics and food safety (e.g., to monitor microbial contamination in food).
MassTag PCR Uses Labelled or Tagged Primers
To label your PCR products with a specific MassTag, you use primers modified at the 5’ end with either nucleotide extensions or commercially available MassTags. For example, the 5’ region can be modified so that each primer pair contains a repeat of thymidines (Ts) for mass differentiation, with each repeat containing a variable number of Ts. Regardless of how your primers are labelled, there are some extra steps that you must follow after completing the PCR.
Processing Steps After the PCR
After a MassTag PCR, products are treated with shrimp alkaline phosphatase and exonuclease 1 to clean up excess primers and nucleotides (4). UV light can cleave many commercially available MassTags from the PCR products, freeing the tag for mass spectrometry. Essentially, you will get quantitative mass spectrometric data where different peaks correspond to the specific barcodes or MassTags (Figure 1).

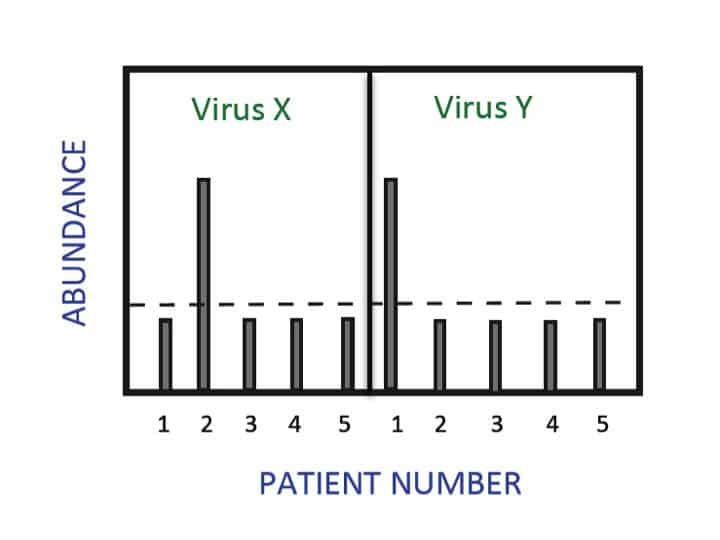
Figure 1. Schematic representation of typical multiplex MassTag PCR result. Here, RNA from 5 patient samples known to contain viral pathogens was subjected to cDNA synthesis and MassTag PCR using appropriately labelled primers. The heights of the bars indicate the abundance of the MassTag (barcode) corresponding to Virus X or Virus Y. The dotted horizontal line represents an arbitrary cut-off value reflecting the average background signal obtained with control human DNA. In this simplified experiment, Patient 2 tested positive for Virus X, while Patient 1 tested positive for Virus Y. In reality, a larger number of viruses would be assayed simultaneously using very little starting material.
How Does MassTag PCR Compare to Conventional PCR?
Advantages
- MassTag PCR is highly sensitive and has the power to detect many more amplicons than is possible with conventional multiplex PCR. Imagine multiplexing 64 different tags (3), so you can test for multiple pathogenic bacteria in one shot! Or getting even more data from extremely limited starting material (e.g., a precious biopsy sample).
- The fact that mass spectrometry can detect size differences down to one base allows you to analyze closely related sequences with high confidence, opening up endless possibilities for your research!
- The set-up is similar to a conventional PCR, so you don’t have to start from scratch! For example, you can use any conventional PCR machine with specially designed primers to run MassTag PCR.
- No more battling with dodgy electrodes on your gel electrophoresis equipment or playing around with toxic DNA stains. And no more second guessing about DNA band sizes! Instead, MassTag PCR gives you a crystal clear picture of what you have based on the presence of the specific MassTags.
Disadvantages
- The preparation of specialized primers may be time-consuming and expensive.
- You will need a mass spectrometer and someone who knows how to use it! This apparatus is still somewhat foreign to biologists. Therefore, you need to learn some basics about mass spectrometry.
The Beauty of MassTag PCR
I have shown you a variation on multiplex PCR called MassTag PCR. The beauty of this technique is its multiplexing ability and accuracy, opening up endless possibilities for you in the lab. So far this technique has proven to be extremely powerful in medical diagnostics. MassTag PCR represents another good example of combining mass spectrometry and biology (can’t beat good ‘ol MALDT-TOF or mass cytometry)!
It is possible that the need for mass spectrometry might slow the popularity of MassTag PCR, but I can’t wait for the day it takes off just like mass cytometry – time of flight!
References
- Haff LA, Smirnov IP. (1997) Multiplex genotyping of PCR products with MassTag-labeled primers. Nucleic Acids Res. 25(18):3749-50. doi:10.1093/nar/25.18.3749.
- Briese T, Palacios G, Kokoris M, Jabado O, Liu Z, Renwick N, et al. (2005) Diagnostic system for rapid and sensitive differential detection of pathogens. Emerg Infect Dis. 11(2):310-3. doi:10.3201/eid1102.040492.
Further Reading
- Doellinger J, Schroeder K, Witt N, Heunemann C, Nitsche A. (2012) Comparison of real-time PCR and MassTag PCR for the multiplex detection of highly pathogenic agents. Mol Cell Probes. 26(5):177-81. doi:10.1016/j.mcp.2012.07.002.
- Palacios G, Briese T, Kapoor V, Jabado O, Liu Z, Venter M, et al. (2006) MassTag polymerase chain reaction for differential diagnosis of viral hemorrhagic fever. Emerg Infect Dis. 12(4):692-5. doi:10.3201/eid1204.051515.