Choosing the Right E. coli Strain for Transformation
Cloning, purifying, and expressing modified genetic material is routinely done in microbes such as Escherichia coli (E.coli). Relatives of this molecular biology workhorse normally live in the intestinal track of humans. The particular E. coli strain (K-12) that scientists use all over the world was isolated from the feces of a diphtheria patient in 1922.1 However, the K-12 strain is largely considered non-pathogenic to humans.
E. coli has become a model system, because it easily and rapidly grows in defined media. In the logarithmic growth phase, these bugs divide every 20 to 30 minutes! Most importantly, E. coli’s ability to take up foreign DNA plasmids (transformability) and its ease of use in making different genetics mutants ultimately landed it an indispensable spot in the “molecular biologist’s toolbox.”
As I have mentioned, there are many different strains of E.coli you can choose depending on the type of experiment you would like to do. Today, I will talk about different aspects of the biology of E.coli, which will provide important parameters when it comes to choosing the right E. coli strain for transformation.
Common Genetic Mutants in E. coli
The first thing you want to find out is the genetic information about the E. coli strain that you will be using. A variety of engineered strains exist to carry certain mutations to suit your experimental needs.
At the most basic level, most of the E. coli we use in the lab contain mutations that restrict its growth in the wild. A common mutation might be in the metabolism compartment. For example, if the strain has a leuB mutation, you will have to add leucine to the media for it to propagate.
Some strains carry the fertility factor (F+). This means they contain an extra episomal plasmid to pass their genetic material to a F- bacterium. F+ strains are required for infection by vector-based filamentous phage because they contain pili on their surface.1
On the other hand, certain common mutations such as tonA confer the strain the ability to resist T1 and T5 phage infection. This can dramatically reduce genetic contaminants during a library construction.2
Important E. coli Machinery
Methylation
The restriction system in E. coli acts like the immune system in humans. As the name implies, the restriction system restricts the propagation of foreign DNAs by destroying them. Certain DNA sites within the E. coli genome are methylated, which means that a methyl group is added to a specific base. Upon cell division, the DNA methylation pattern is inherited by E. coli daughter cells. Because foreign DNA does not undergo this process and remains unmethylated, the restriction system has a basis for identifying foreign DNA. Therefore, the enzymes that perform methylation, the E. coli methylases and the endonucleases that cut the unmethylated DNA, play an important role in the organism’s defense.
The E. coli K-12 methylase system commonly consists of three different types of methylases: Dam, Dcm, and Eco K.1 The methylases transfer a methyl group to an adenine or a cytosine base within a recognition motif or site in the genome, thus effectively labeling the native E. coli genome as “self”. The E. coli endonucleases recognize DNA specific recognition sites and cleave the DNA within those sites. The endonculease system is prevented from cleaving methylated restriction sites. Therefore, foreign DNA that lacks this methylation can be cleaved by the native endonculeases.
Because certain endonucleases are prevented from digesting methylated DNA, choose a dam and dcm deficient E. coli strain if you want to prepare plasmids that are cut by endonucleases at dam methylated sites!
Recombination
Recombination is important in bacteria because it allows them to “re-shuffle” their genetic material and boost their chance of survival. However, a molecular biologist’s worst nightmare is to realize his “favorite” plasmid/protein sequence has been scrambled due to natural selection! Recombination is especially a big problem if the cloned sequence contains direct repeats, or if you are constructing a genetic library.
Therefore, most of the common laboratory strains contain mutations in genes that encode proteins in the recombination pathway. There are three major recombination pathway: recBCD, recE, and recF. All of these pathways fall under the master switch called recA. That’s why you will find a lot of strains that are recA-. The mutation in recA can lower the rate of recombination by 10,000 fold! However, this particular mutation makes the bacteria very crippled and difficult to grow.
Important Modifications of E. coli
Blue/White selection
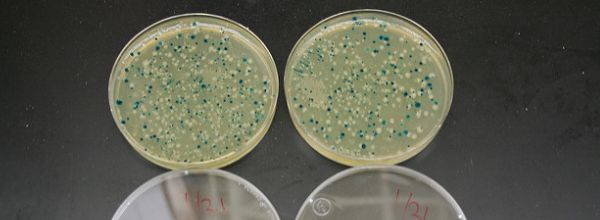
One of the important modifications to E.coli that allows visual screening of colonies came from the study of the lac operon. The bacterial operon contains three proteins that work in concert to break down lactose into glucose and galactose. The lac operon is controlled by a “de-repressor”, which binds to lactose. So, the operon is only turned on in the presence of lactose, which allows any molecular biologist to tightly control expression of genes within the lac operon.
The key player is the lacZ gene that encodes beta-galactosidase, a permease and transacetylase. Mutations in either the 5’ (alpha-fragment) or the 3’ (w-fragment) of the beta-galactosidase protein make it inactive. Wait, what if you separate the two fragments and then bring them together?
Bingo! You get normal beta-galactosidase activity.
This is called alpha-complementation and forms the basis of blue and white selection. The blue/white selection plasmid contains the gene for the alpha-fragment, which is deleted from the E. coli genome. Then a multiple cloning site (MCS) is inserted in the middle of the alpha-fragment gene in the plasmid. So, when you insert your gene of interest into the MCS, alpha-complementation will be inhibited, beta-galactosidase can’t break down lactose into glucose and galactose, and the colonies will be white. If your gene isn’t present within the alpha-fragment gene, then alpha-complementation will occur and the colonies will be blue. You can pick the white colonies and screen for your insert. This complementation assay is a great way to screen for your inserted gene.
IPTG Induction for Protein Expression
Similarly, the lac operon can be engineered to regulate gene and protein expression. For example, the pET expression system contains a lacI gene, which codes for the lac repressor and a T7 promoter that is specific for T7 RNA polymerase. The pET plasmid is a great protein expression system and is greatly complemented by E.coli strains deficient in protease.
Conclusion
I hope this background on E. coli strains will help you make a better choice for your experiments. You can also gain more appreciation for what this little bug can do and the potential of molecular biology!
References
- Casali, Nicola. Escherichia Coli Host Strains. In E. Coli Plasmid Vectors, edited by Nicola Casali and Andrew Preston, 27–48. Methods in Molecular Biology. 235. Humana Press, 2003.
- Plasmids 101: Common Lab E. Coli Strains. Accessed December 21, 2015.
1 Comments
Leave a Reply Cancel Reply
You must be logged in to post a comment.
Hi I’m having trouble to devise an experiment to carry out this task: An insert is available containing a gene within it whose orientation and exact position with the insert are unknown. The gene contains a sequence for an important small peptide hormone. No DNA sequence info is available. Set up an experimental scheme that takes into account the task described above, beginning with the insert and the expression vector pUC18. A map of pUC18 with all features indicated, including the multi-cloning site, may be easily obtained by typing the vector name into Google. The insert containing the peptide gene is a blunt end fragment of 1.8kb that was not generated with a restriction enzyme. The gene does not have a promoter and there is 6* his tag at the C-terminus of the open reading frame.